Dietary Supplementation With Medium-Chain Triglycerides Reduces Candida Gastrointestinal Colonization in Preterm Infants
- PMID: 29596218
- PMCID: PMC6604858
- DOI: 10.1097/INF.0000000000002042
Dietary Supplementation With Medium-Chain Triglycerides Reduces Candida Gastrointestinal Colonization in Preterm Infants
Abstract
Background: Candida is an important cause of infections in premature infants. Gastrointestinal colonization with Candida is a common site of entry for disseminated disease. The objective of this study was to determine whether a dietary supplement of medium-chain triglycerides (MCTs) reduces Candida colonization in preterm infants.
Methods: Preterm infants with Candida colonization (n = 12) receiving enteral feedings of either infant formula (n = 5) or breast milk (n = 7) were randomized to MCT supplementation (n = 8) or no supplementation (n = 4). Daily stool samples were collected to determine fungal burden during a 3-week study period. Infants in the MCT group received supplementation during 1 week of the study period. The primary outcome was fungal burden during the supplementation period as compared with the periods before and after supplementation.
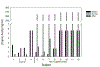
Results: Supplementation of MCT led to a marked increase in MCT intake relative to unsupplemented breast milk or formula as measured by capric acid content. In the treatment group, there was a significant reduction in fungal burden during the supplementation period as compared with the period before supplementation (rate ratio, 0.15; P = 0.02), with a significant increase after supplementation was stopped (rate ratio, 61; P < 0.001). Fungal burden in the control group did not show similar changes.
Conclusions: Dietary supplementation with MCT may be an effective method to reduce Candida colonization in preterm infants.
Figures

Similar articles
-
Minimal Effects of Medium-Chain Triglyceride Supplementation on the Intestinal Microbiome Composition of Premature Infants: A Single-Center Pilot Study.Nutrients. 2022 May 22;14(10):2159. doi: 10.3390/nu14102159. Nutrients. 2022. PMID: 35631300 Free PMC article. Clinical Trial.
-
Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study.Clin Infect Dis. 2006 Jun 15;42(12):1735-42. doi: 10.1086/504324. Epub 2006 May 4. Clin Infect Dis. 2006. PMID: 16705580 Clinical Trial.
-
Incidence of Candida parapsilosis colonization in an intensive care nursery population and its association with invasive fungal disease.Pediatr Infect Dis J. 1994 Jun;13(6):520-4. doi: 10.1097/00006454-199406000-00011. Pediatr Infect Dis J. 1994. PMID: 8078741
-
Use of Lactobacillus casei subspecies Rhamnosus GG and gastrointestinal colonization by Candida species in preterm neonates.J Pediatr Gastroenterol Nutr. 2007 Dec;45 Suppl 3:S190-4. doi: 10.1097/01.mpg.0000302971.06115.15. J Pediatr Gastroenterol Nutr. 2007. PMID: 18185091 Review.
-
Enteral and parenteral lipid requirements of preterm infants.World Rev Nutr Diet. 2014;110:82-98. doi: 10.1159/000358460. Epub 2014 Apr 11. World Rev Nutr Diet. 2014. PMID: 24751623 Review.
Cited by
-
The Effect of Medium-Chain Triglycerides Oil on Candida albicans and Streptococcus mutans in Planktonic and Mucosal Models.Antibiotics (Basel). 2024 Dec 20;13(12):1231. doi: 10.3390/antibiotics13121231. Antibiotics (Basel). 2024. PMID: 39766621 Free PMC article.
-
Minimal Effects of Medium-Chain Triglyceride Supplementation on the Intestinal Microbiome Composition of Premature Infants: A Single-Center Pilot Study.Nutrients. 2022 May 22;14(10):2159. doi: 10.3390/nu14102159. Nutrients. 2022. PMID: 35631300 Free PMC article. Clinical Trial.
-
Partridge and embryonated partridge egg as new preclinical models for candidiasis.Sci Rep. 2021 Jan 22;11(1):2072. doi: 10.1038/s41598-021-81592-y. Sci Rep. 2021. PMID: 33483560 Free PMC article.
-
Eligibility Criteria and Representativeness of Randomized Clinical Trials That Include Infants Born Extremely Premature: A Systematic Review.J Pediatr. 2021 Aug;235:63-74.e12. doi: 10.1016/j.jpeds.2021.04.028. Epub 2021 Apr 21. J Pediatr. 2021. PMID: 33894262 Free PMC article.
-
High versus low medium chain triglyceride content of formula for promoting short-term growth of preterm infants.Cochrane Database Syst Rev. 2021 Feb 23;2(2):CD002777. doi: 10.1002/14651858.CD002777.pub2. Cochrane Database Syst Rev. 2021. PMID: 33620090 Free PMC article.
References
-
- Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. - PubMed
-
- Benjamin DK Jr., Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. - PubMed
-
- Manzoni P, Farina D, Leonessa M, et al. Risk factors for progression to invasive fungal infection in preterm neonates with fungal colonization. Pediatrics. 2006;118:2359–2364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

