Differences in the Effects of EGCG on Chromosomal Stability and Cell Growth between Normal and Colon Cancer Cells
- PMID: 29596305
- PMCID: PMC6017350
- DOI: 10.3390/molecules23040788
Differences in the Effects of EGCG on Chromosomal Stability and Cell Growth between Normal and Colon Cancer Cells
Abstract
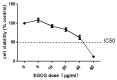
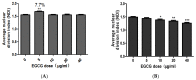
The tea catechin epigallocatechin-3-gallate (EGCG) proved to be the most potent physiologically active tea compound in vitro. It possesses antioxidant as well as pro-oxidant properties. EGCG has the effect of inducing apoptosis of tumor cells and inhibiting cell proliferation. Whether this effect is associated with the antioxidant or pro-oxidative effects of EGCG affecting the genome stability of normal and cancer cells has not been confirmed. Here, we selected Human normal colon epithelial cells NCM460 and colon adenocarcinoma cells COLO205 to investigate the effects of EGCG (0−40 μg/mL) on the genome stability and cell growth status. Chromosomal instability (CIN), nuclear division index (NDI), and apoptosis was measured by cytokinesis-block micronucleus assay (CBMN), and the expression of core genes in mismatch repair (hMLMLH1 and hMSH2) was examined by RT-qPCR. We found that EGCG significantly reduced CIN and apoptosis rate of NCM460 at all concentrations (5−40 μg/mL) and treatment time, EGCG at 5 μg/mL promoted cell division; EGCG could significantly induce chromosome instability in COLO205 cells and trigger apoptosis and inhibition of cell division. These results suggest that EGCG exhibits different genetic and cytological effects in normal and colon cancer cells.
Keywords: apoptosis; chromosomal instability (CIN); epigallocatechin-3-gallate (EGCG); human colon cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



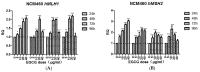
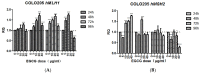
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

