Porous Polyethylene Coated with Functionalized Hydroxyapatite Particles as a Bone Reconstruction Material
- PMID: 29596358
- PMCID: PMC5951367
- DOI: 10.3390/ma11040521
Porous Polyethylene Coated with Functionalized Hydroxyapatite Particles as a Bone Reconstruction Material
Abstract
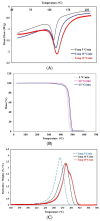
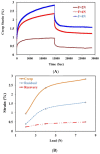
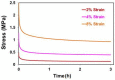
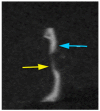
In this study, porous polyethylene scaffolds were examined as bone substitutes in vitro and in vivo in critical-sized calvarial bone defects in transgenic Sprague-Dawley rats. A microscopic examination revealed that the pores appeared to be interconnected across the material, making them suitable for cell growth. The creep recovery behavior of porous polyethylene at different loads indicated that the creep strain had two main portions. In both portions, strain increased with increased applied load and temperature. In terms of the thermographic behavior of the material, remarkable changes in melting temperature and heat fusion were revealed with increased the heating rates. The tensile strength results showed that the material was sensitive to the strain rate and that there was adequate mechanical strength to support cell growth. The in vitro cell culture results showed that human bone marrow mesenchymal stem cells attached to the porous polyethylene scaffold. Calcium sulfate-hydroxyapatite (CS-HA) coating of the scaffold not only improved attachment but also increased the proliferation of human bone marrow mesenchymal stem cells. In vivo, histological analysis showed that the study groups had active bone remodeling at the border of the defect. Bone regeneration at the border was also evident, which confirmed that the polyethylene acted as an osteoconductive bone graft. Furthermore, bone formation inside the pores of the coated polyethylene was also noted, which would enhance the process of osteointegration.
Keywords: hydroxyapatite; mesenchymal stem cells; porous polyethylene.
Conflict of interest statement
The authors declare that there are no conflicts of interest or state among all the contributors.
Figures










References
-
- Behnaz B., Payam Z., Mohammad O.O., Farid K., Hamideh F., Sohrabi-Jahromi S., Zarrintaj Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017;33:144–172. - PubMed
-
- Schlickewei W., Schlickewei S. The use of bone substitutes in the treatment of bone defects—The clinical view and history. Macromol. Symp. 2007;253:10–23. doi: 10.1002/masy.200750702. - DOI
-
- Stevens M.M. Biomaterials for bone tissue engineering. Mater. Today. 2008;11:18–25. doi: 10.1016/S1369-7021(08)70086-5. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

