Optimal Serum Ferritin Levels for Iron Deficiency Anemia during Oral Iron Therapy (OIT) in Japanese Hemodialysis Patients with Minor Inflammation and Benefit of Intravenous Iron Therapy for OIT-Nonresponders
- PMID: 29596361
- PMCID: PMC5946213
- DOI: 10.3390/nu10040428
Optimal Serum Ferritin Levels for Iron Deficiency Anemia during Oral Iron Therapy (OIT) in Japanese Hemodialysis Patients with Minor Inflammation and Benefit of Intravenous Iron Therapy for OIT-Nonresponders
Abstract
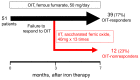
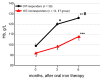
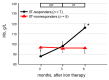
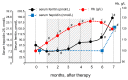
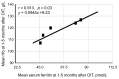
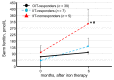
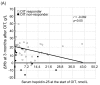
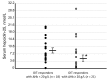
Background: We determined optimal serum ferritin for oral iron therapy (OIT) in hemodialysis (HD) patients with iron deficiency anemia (IDA)/minor inflammation, and benefit of intravenous iron therapy (IIT) for OIT-nonresponders. Methods: Inclusion criteria were IDA (Hb <120 g/L, serum ferritin <227.4 pmol/L). Exclusion criteria were inflammation (C-reactive protein (CRP) ≥ 5 mg/L), bleeding, or cancer. IIT was withheld >3 months before the study. ΔHb ≥ 20 g/L above baseline or maintaining target Hb (tHB; 120-130 g/L) was considered responsive. Fifty-one patients received OIT (ferrous fumarate, 50 mg/day) for 3 months; this continued in OIT-responders but was switched to IIT (saccharated ferric oxide, 40 mg/week) in OIT-nonresponders for 4 months. All received continuous erythropoietin receptor activator (CERA). Hb, ferritin, hepcidin-25, and CERA dose were measured. Results: Demographics before OIT were similar between OIT-responders and OIT-nonresponders except low Hb and high triglycerides in OIT-nonresponders. Thirty-nine were OIT-responders with reduced CERA dose. Hb rose with a peak at 5 months. Ferritin and hepcidin-25 continuously increased. Hb positively correlated with ferritin in OIT-responders (r = 0.913, p = 0.03) till 5 months after OIT. The correlation equation estimated optimal ferritin of 30-40 ng/mL using tHb (120-130 g/L). Seven OIT-nonresponders were IIT-responders. Conclusions: Optimal serum ferritin for OIT is 67.4-89.9 pmol/L in HD patients with IDA/minor inflammation. IIT may be a second line of treatment for OIT-nonreponders.
Keywords: ferritin; hemodialysis; hepcidin-25; inflammation; iron deficiency anemia; oral iron therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- The Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012;2:279–335.
-
- Macdougall I.C., Bock A.H., Carrera F., Eckardt K.U., Gaillard C., Van Wyck D., Roubert B., Nolen J.G., Roger S.D., FIND-CKD Study Investigators FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transplant. 2014;29:2075–2084. doi: 10.1093/ndt/gfu201. - DOI - PMC - PubMed
-
- Tsubakihara Y., Nishi S., Akiba T., Hirakata H., Iseki K., Kubota M., Kuriyama S., Komatsu Y., Suzuki M., Nakai S., et al. 2008 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ther. Apher. Dial. 2010;14:240–275. doi: 10.1111/j.1744-9987.2010.00836.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

