Carbene Transfer Reactions Catalysed by Dyes of the Metalloporphyrin Group
- PMID: 29596367
- PMCID: PMC6017490
- DOI: 10.3390/molecules23040792
Carbene Transfer Reactions Catalysed by Dyes of the Metalloporphyrin Group
Abstract
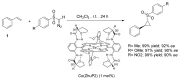
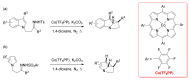
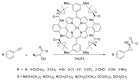
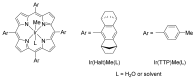
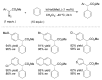
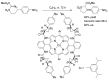
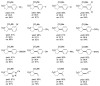
Carbene transfer reactions are very important transformations in organic synthesis, allowing the generation of structurally challenging products by catalysed cyclopropanation, cyclopropenation, carbene C-H, N-H, O-H, S-H, and Si-H insertion, and olefination of carbonyl compounds. In particular, chiral and achiral metalloporphyrins have been successfully explored as biomimetic catalysts for these carbene transfer reactions under both homogeneous and heterogeneous conditions. In this work the use of synthetic metalloporphyrins (MPorph, M = Fe, Ru, Os, Co, Rh, Ir, Sn) as homogeneous or heterogeneous catalysts for carbene transfer reactions in the last years is reviewed, almost exclusively focused on the literature since the year 2010, except when reference to older publications was deemed to be crucial.
Keywords: X-H insertion; carbenes; cyclopropanation; cyclopropenation; metalloporphyrins; olefination; porphyrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

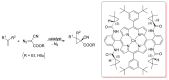

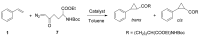
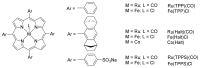
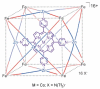
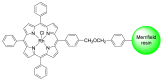
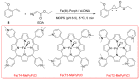
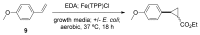
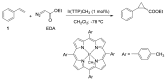
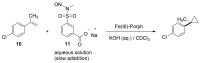
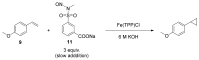
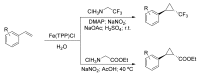
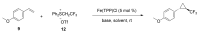
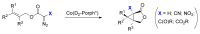
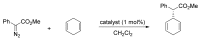
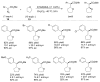
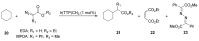
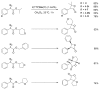
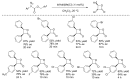
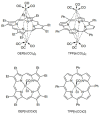
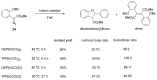
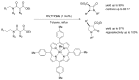
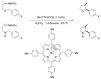
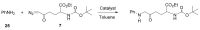
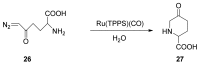

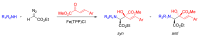
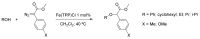
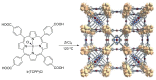
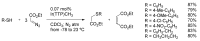


References
-
- IUPAC . Compendium of Chemical Terminology. 2nd ed. Blackwell Scientific Publications; Oxford, UK: 2014. [(accessed on 29 March 2018)]. The “Gold Book”. Available online: https://goldbook.iupac.org/html/C/C00806.html.
-
- Chakraborty J., Nath I., Verpoort F. Snapshots of encapsulated porphyrins and heme enzymes in metal-organic materials: A prevailing paradigm of heme mimicry. Coord. Chem. Rev. 2016;326:135–163. doi: 10.1016/j.ccr.2016.08.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

