Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847
- PMID: 29596370
- PMCID: PMC5923398
- DOI: 10.3390/md16040111
Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia reniformis Nardo, 1847
Abstract
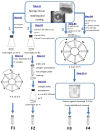
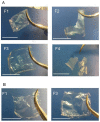
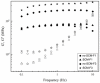
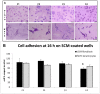
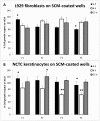
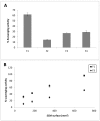
Collagen is involved in the formation of complex fibrillar networks, providing the structural integrity of tissues. Its low immunogenicity and mechanical properties make this molecule a biomaterial that is extremely suitable for tissue engineering and regenerative medicine (TERM) strategies in human health issues. Here, for the first time, we performed a thorough screening of four different methods to obtain sponge collagenous fibrillar suspensions (FSs) from C. reniformis demosponge, which were then chemically, physically, and biologically characterized, in terms of protein, collagen, and glycosaminoglycans content, viscous properties, biocompatibility, and antioxidant activity. These four FSs were then tested for their capability to generate crosslinked or not thin sponge collagenous membranes (SCMs) that are suitable for TERM purposes. Two types of FSs, of the four tested, were able to generate SCMs, either from crosslinking or not, and showed good mechanical properties, enzymatic degradation resistance, water binding capacity, antioxidant activity, and biocompatibility on both fibroblast and keratinocyte cell cultures. Finally, our results demonstrate that it is possible to adapt the extraction procedure in order to alternatively improve the mechanical properties or the antioxidant performances of the derived biomaterial, depending on the application requirements, thanks to the versatility of C. reniformis extracellular matrix extracts.
Keywords: Porifera; biomaterials; collagen; membranes; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
2D Collagen Membranes from Marine Demosponge Chondrosia reniformis (Nardo, 1847) for Skin-Regenerative Medicine Applications: An In Vitro Evaluation.Mar Drugs. 2023 Jul 28;21(8):428. doi: 10.3390/md21080428. Mar Drugs. 2023. PMID: 37623709 Free PMC article.
-
Bioinspiring Chondrosia reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material.Mar Drugs. 2017 Dec 4;15(12):380. doi: 10.3390/md15120380. Mar Drugs. 2017. PMID: 29207538 Free PMC article.
-
Seasonal Molecular Difference in Fibrillar Collagen Extracts Derived from the Marine Sponge Chondrosia reniformis (Nardo, 1847) and Their Impact on Its Derived Biomaterials.Mar Drugs. 2023 Mar 28;21(4):210. doi: 10.3390/md21040210. Mar Drugs. 2023. PMID: 37103350 Free PMC article.
-
Processing of collagen based biomaterials and the resulting materials properties.Biomed Eng Online. 2019 Mar 18;18(1):24. doi: 10.1186/s12938-019-0647-0. Biomed Eng Online. 2019. PMID: 30885217 Free PMC article. Review.
-
Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications.Tissue Eng Part B Rev. 2020 Apr;26(2):164-180. doi: 10.1089/ten.TEB.2019.0256. Epub 2020 Feb 4. Tissue Eng Part B Rev. 2020. PMID: 31910095 Review.
Cited by
-
The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia reniformis (Nardo, 1847).Mar Drugs. 2020 Jul 10;18(7):358. doi: 10.3390/md18070358. Mar Drugs. 2020. PMID: 32664196 Free PMC article.
-
Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates.Molecules. 2020 May 26;25(11):2471. doi: 10.3390/molecules25112471. Molecules. 2020. PMID: 32466475 Free PMC article. Review.
-
On the Path to Thermo-Stable Collagen: Culturing the Versatile Sponge Chondrosia reniformis.Mar Drugs. 2021 Nov 26;19(12):669. doi: 10.3390/md19120669. Mar Drugs. 2021. PMID: 34940668 Free PMC article.
-
2D Collagen Membranes from Marine Demosponge Chondrosia reniformis (Nardo, 1847) for Skin-Regenerative Medicine Applications: An In Vitro Evaluation.Mar Drugs. 2023 Jul 28;21(8):428. doi: 10.3390/md21080428. Mar Drugs. 2023. PMID: 37623709 Free PMC article.
-
Potential of Marine Biomolecules: Advances in Extraction and Applications of Proteins, Polysaccharides, and Antioxidant Compounds.Foods. 2025 Jul 22;14(15):2555. doi: 10.3390/foods14152555. Foods. 2025. PMID: 40807492 Free PMC article. Review.
References
-
- Buijtenhuijs P., Buttafoco L., Poot A.A., Daamen W.F., van Kuppevelt T.H., Dijkstra P., de Vos R.A., Ster L.M., Geelkerken B.R., Feijen J., et al. Tissue engineering of bloodvessels: Characterization of smooth-muscle cells for culturing on collagen-and-elastin-based scaffolds. Biotechnol. Appl. Biochem. 2004;39:141–149. doi: 10.1042/BA20030105. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

