The Role of Serotonin during Skin Healing in Post-Thermal Injury
- PMID: 29596386
- PMCID: PMC5979562
- DOI: 10.3390/ijms19041034
The Role of Serotonin during Skin Healing in Post-Thermal Injury
Abstract
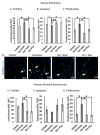
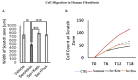
Post-burn trauma significantly raises tissue serotonin concentration at the initial stages of injury, which leads us to investigate its possible role in post burn wound healing. Therefore, we planned this study to examine the role of serotonin in wound healing through in vitro and in vivo models of burn injuries. Results from in vitro analysis revealed that serotonin decreased apoptosis and increased cell survival significantly in human fibroblasts and neonatal keratinocytes. Cellular proliferation also increased significantly in both cell types. Moreover, serotonin stimulation significantly accelerated the cell migration, resulting in narrowing of the scratch zone in human neonatal keratinocytes and fibroblasts cultures. Whereas, fluoxetine (a selective serotonin reuptake inhibitor) and ketanserin (serotonin receptor 2A inhibitor) reversed these effects. Scald burn mice model (20% total body surface area) showed that endogenous serotonin improved wound healing process in control group, whereas fluoxetine and ketanserin treatments (disruptors of endogenous serotonin stimulation), resulted in poor reepithelization, bigger wound size and high alpha smooth muscle actin (α-SMA) count. All of these signs refer a prolonged differentiation state, which ultimately exhibits poor wound healing outcomes. Collectively, data showed that the endogenous serotonin pathway contributes to regulating the skin wound healing process. Hence, the results of this study signify the importance of serotonin as a potential therapeutic candidate for enhancing skin healing in burn patients.
Keywords: fibroblasts; keratinocytes; migration; post thermal injury; serotonin; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- World Health Organization . A WHO Plan for Burn Prevention and Care. World Health Organization; Geneva, Switzerland: 2008. 23p
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

