Development and evaluation of antibody-capture immunoassays for detection of Lassa virus nucleoprotein-specific immunoglobulin M and G
- PMID: 29596412
- PMCID: PMC5892945
- DOI: 10.1371/journal.pntd.0006361
Development and evaluation of antibody-capture immunoassays for detection of Lassa virus nucleoprotein-specific immunoglobulin M and G
Abstract
Background: The classical method for detection of Lassa virus-specific antibodies is the immunofluorescence assay (IFA) using virus-infected cells as antigen. However, IFA requires laboratories of biosafety level 4 for assay production and an experienced investigator to interpret the fluorescence signals. Therefore, we aimed to establish and evaluate enzyme-linked immunosorbent assays (ELISA) using recombinant Lassa virus nucleoprotein (NP) as antigen.
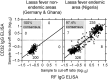
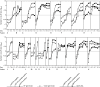
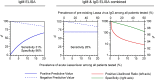
Methodology/principal findings: The IgM ELISA is based on capturing IgM antibodies using anti-IgM, and the IgG ELISA is based on capturing IgG antibody-antigen complexes using rheumatoid factor or Fc gamma receptor CD32a. Analytical and clinical evaluation was performed with 880 sera from Lassa fever endemic (Nigeria) and non-endemic (Ghana and Germany) areas. Using the IFA as reference method, we observed 91.5-94.3% analytical accuracy of the ELISAs in detecting Lassa virus-specific antibodies. Evaluation of the ELISAs for diagnosis of Lassa fever on admission to hospital in an endemic area revealed a clinical sensitivity for the stand-alone IgM ELISA of 31% (95% CI 25-37) and for combined IgM/IgG detection of 26% (95% CI 21-32) compared to RT-PCR. The specificity of IgM and IgG ELISA was estimated at 96% (95% CI 93-98) and 100% (95% CI 99-100), respectively, in non-Lassa fever patients from non-endemic areas. In patients who seroconverted during follow-up, Lassa virus-specific IgM and IgG developed simultaneously rather than sequentially. Consistent with this finding, isolated IgM reactivity, i.e. IgM in the absence of IgG, had no diagnostic value.
Conclusions/significance: The ELISAs are not equivalent to RT-PCR for early diagnosis of Lassa fever; however, they are of value in diagnosing patients at later stage. The IgG ELISA may be useful for epidemiological studies and clinical trials due its high specificity, and the higher throughput rate and easier operation compared to IFA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Sensitivity and specificity of diagnostic tests for Lassa fever: a systematic review.BMC Infect Dis. 2019 Jul 19;19(1):647. doi: 10.1186/s12879-019-4242-6. BMC Infect Dis. 2019. PMID: 31324229 Free PMC article.
-
Reverse ELISA for IgG and IgM antibodies to detect Lassa virus infections in Africa.J Clin Virol. 2006 Dec;37(4):277-81. doi: 10.1016/j.jcv.2006.08.015. Epub 2006 Sep 25. J Clin Virol. 2006. PMID: 16996789
-
Diagnosis and clinical virology of Lassa fever as evaluated by enzyme-linked immunosorbent assay, indirect fluorescent-antibody test, and virus isolation.J Clin Microbiol. 2000 Jul;38(7):2670-7. doi: 10.1128/JCM.38.7.2670-2677.2000. J Clin Microbiol. 2000. PMID: 10878062 Free PMC article.
-
Detection of Lassa virus antinucleoprotein immunoglobulin G (IgG) and IgM antibodies by a simple recombinant immunoblot assay for field use.J Clin Microbiol. 1998 Nov;36(11):3143-8. doi: 10.1128/JCM.36.11.3143-3148.1998. J Clin Microbiol. 1998. PMID: 9774554 Free PMC article.
-
Serological assays based on recombinant viral proteins for the diagnosis of arenavirus hemorrhagic fevers.Viruses. 2012 Oct 12;4(10):2097-114. doi: 10.3390/v4102097. Viruses. 2012. PMID: 23202455 Free PMC article. Review.
Cited by
-
Fcγ-Receptor-Based Enzyme-Linked Immunosorbent Assays for Sensitive, Specific, and Persistent Detection of Anti-SARS-CoV-2 Nucleocapsid Protein IgG Antibodies in Human Sera.J Clin Microbiol. 2022 Jun 15;60(6):e0007522. doi: 10.1128/jcm.00075-22. Epub 2022 May 16. J Clin Microbiol. 2022. PMID: 35574677 Free PMC article.
-
Lassa Virus Countermeasures.Curr Top Microbiol Immunol. 2023;440:111-145. doi: 10.1007/82_2022_261. Curr Top Microbiol Immunol. 2023. PMID: 36253593
-
Seroepidemiology of Lassa virus in pregnant women in Southern Nigeria: A prospective hospital-based cohort study.PLoS Negl Trop Dis. 2023 May 22;17(5):e0011354. doi: 10.1371/journal.pntd.0011354. eCollection 2023 May. PLoS Negl Trop Dis. 2023. PMID: 37216412 Free PMC article.
-
Validation of the Particle-Based Multi-Analyte Technology for Detection of Anti-PhosphatidylSerine/Prothrombin Antibodies.Biomedicines. 2020 Dec 17;8(12):622. doi: 10.3390/biomedicines8120622. Biomedicines. 2020. PMID: 33348782 Free PMC article.
-
Sensitivity and specificity of diagnostic tests for Lassa fever: a systematic review.BMC Infect Dis. 2019 Jul 19;19(1):647. doi: 10.1186/s12879-019-4242-6. BMC Infect Dis. 2019. PMID: 31324229 Free PMC article.
References
-
- Frame JD, Baldwin JM Jr., Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19(4): 670–6. - PubMed
-
- Carey DE, Kemp GE, White HA, Pinneo L, Addy RF, Fom AL, et al. Lassa fever. Epidemiological aspects of the 1970 epidemic, Jos, Nigeria. Trans R Soc Trop Med Hyg. 1972;66(3): 402–8. - PubMed
-
- Monath TP, Mertens PE, Patton R, Moser CR, Baum JJ, Pinneo L, et al. A hospital epidemic of Lassa fever in Zorzor, Liberia, March-April 1972. Am J Trop Med Hyg. 1973;22(6): 773–9. - PubMed
-
- McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155(3): 437–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

