The colonic epithelium plays an active role in promoting colitis by shaping the tissue cytokine profile
- PMID: 29596476
- PMCID: PMC5892915
- DOI: 10.1371/journal.pbio.2002417
The colonic epithelium plays an active role in promoting colitis by shaping the tissue cytokine profile
Abstract
Inflammatory bowel disease (IBD) is a chronic condition driven by loss of homeostasis between the mucosal immune system, the commensal gut microbiota, and the intestinal epithelium. Our goal is to understand how these components of the intestinal ecosystem cooperate to control homeostasis. By combining quantitative measures of epithelial hyperplasia and immune infiltration with multivariate analysis of inter- and intracellular signaling, we identified epithelial mammalian target of rapamycin (mTOR) signaling as a potential driver of inflammation in a mouse model of colitis. A kinetic analysis of mTOR inhibition revealed that the pathway regulates epithelial differentiation, which in turn controls the cytokine milieu of the colon. Consistent with our in vivo analysis, we found that cytokine expression of organoids grown ex vivo, in the absence of bacteria and immune cells, was dependent on differentiation state. Our study suggests that proper differentiation of epithelial cells is an important feature of colonic homeostasis because of its effect on the secretion of inflammatory cytokines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


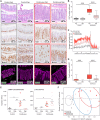
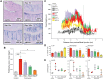
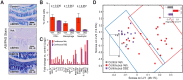


References
-
- Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60(5):571–607. doi: 10.1136/gut.2010.224154 . - DOI - PubMed
-
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. doi: 10.1038/nature11582 . - DOI - PMC - PubMed
-
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–17. doi: 10.1038/nature10209 . - DOI - PMC - PubMed
-
- Roda G, Sartini A, Zambon E, Calafiore A, Marocchi M, Caponi A, et al. Intestinal epithelial cells in inflammatory bowel diseases. World J Gastroenterol. 2010;16(34):4264–71. doi: 10.3748/wjg.v16.i34.4264 . - DOI - PMC - PubMed
-
- Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53. doi: 10.1038/nri3608 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

