Characterisation of insulin analogues therapeutically available to patients
- PMID: 29596514
- PMCID: PMC5875863
- DOI: 10.1371/journal.pone.0195010
Characterisation of insulin analogues therapeutically available to patients
Abstract
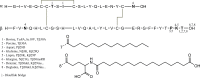
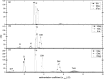
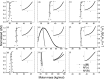
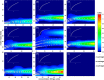
The structure and function of clinical dosage insulin and its analogues were assessed. This included 'native insulins' (human recombinant, bovine, porcine), 'fast-acting analogues' (aspart, glulisine, lispro) and 'slow-acting analogues' (glargine, detemir, degludec). Analytical ultracentrifugation, both sedimentation velocity and equilibrium experiments, were employed to yield distributions of both molar mass and sedimentation coefficient of all nine insulins. Size exclusion chromatography, coupled to multi-angle light scattering, was also used to explore the function of these analogues. On ultracentrifugation analysis, the insulins under investigation were found to be in numerous conformational states, however the majority of insulins were present in a primarily hexameric conformation. This was true for all native insulins and two fast-acting analogues. However, glargine was present as a dimer, detemir was a multi-hexameric system, degludec was a dodecamer (di-hexamer) and glulisine was present as a dimer-hexamer-dihexamer system. However, size-exclusion chromatography showed that the two hexameric fast-acting analogues (aspart and lispro) dissociated into monomers and dimers due to the lack of zinc in the mobile phase. This comprehensive study is the first time all nine insulins have been characterised in this way, the first time that insulin detemir have been studied using analytical ultracentrifugation and the first time that insulins aspart and glulisine have been studied using sedimentation equilibrium. The structure and function of these clinically administered insulins is of critical importance and this research adds novel data to an otherwise complex functional physiological protein.
Conflict of interest statement
Figures

Similar articles
-
Identification of recombinant human insulin and biosynthetic insulin analogues by multiplexed targeted unlabeled mass spectrometry of proteotypic tryptic peptides.J Pharm Biomed Anal. 2019 Aug 5;172:357-363. doi: 10.1016/j.jpba.2019.04.052. Epub 2019 May 1. J Pharm Biomed Anal. 2019. PMID: 31096094
-
Development and validation of a method for quantification of human insulin and its synthetic analogues in plasma and post-mortem sera by LC-MS/HRMS.Talanta. 2021 Apr 1;225:122047. doi: 10.1016/j.talanta.2020.122047. Epub 2020 Dec 24. Talanta. 2021. PMID: 33592769
-
Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature.J Pharm Sci. 2014 Aug;103(8):2255-67. doi: 10.1002/jps.24039. Epub 2014 Jun 6. J Pharm Sci. 2014. PMID: 24909933
-
Safety of insulin analogues as compared with human insulin in pregnancy.Expert Opin Drug Saf. 2016 Jul;15(7):963-73. doi: 10.1080/14740338.2016.1182153. Epub 2016 May 17. Expert Opin Drug Saf. 2016. PMID: 27145344 Review.
-
Pharmacotherapy for hyperglycemia in pregnancy - The new insulins.Diabetes Res Clin Pract. 2018 Nov;145:59-66. doi: 10.1016/j.diabres.2018.04.035. Epub 2018 May 3. Diabetes Res Clin Pract. 2018. PMID: 29730391 Review.
Cited by
-
Biopolymeric Insulin Membranes for Antimicrobial, Antioxidant, and Wound Healing Applications.Pharmaceutics. 2024 Jul 30;16(8):1012. doi: 10.3390/pharmaceutics16081012. Pharmaceutics. 2024. PMID: 39204356 Free PMC article.
-
The Other Face of Insulin-Overdose and Its Effects.Toxics. 2022 Mar 3;10(3):123. doi: 10.3390/toxics10030123. Toxics. 2022. PMID: 35324747 Free PMC article. Review.
-
Exchange Broadening Underlies the Enhancement of IDE-Dependent Degradation of Insulin by Anionic Membranes.ACS Omega. 2022 Jul 7;7(28):24757-24765. doi: 10.1021/acsomega.2c02747. eCollection 2022 Jul 19. ACS Omega. 2022. PMID: 35874268 Free PMC article.
-
Open-source 3-D printable autoinjector: Design, testing, and regulatory limitations.PLoS One. 2023 Jul 14;18(7):e0288696. doi: 10.1371/journal.pone.0288696. eCollection 2023. PLoS One. 2023. PMID: 37450496 Free PMC article.
-
Measuring the Mechanical Properties of Insulin: A Potential Solution to Overcoming the Challenges of Real-Time, Point-of-Care Insulin Sensing.J Diabetes Sci Technol. 2025 Apr 9:19322968251331072. doi: 10.1177/19322968251331072. Online ahead of print. J Diabetes Sci Technol. 2025. PMID: 40200746 Free PMC article.
References
-
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. Rockefeller University Press; 2008;183: 1235–1242. doi: 10.1083/jcb.200809113 - DOI - PMC - PubMed
-
- Adams GG, Alzahrani Q, Jiwani SI, Meal A, Morgan PS, Coffey F, et al. Glargine and degludec: Solution behaviour of higher dose synthetic insulins. Sci Rep. Nature Publishing Group; 2017;7: 7287 doi: 10.1038/s41598-017-06642-w - DOI - PMC - PubMed
-
- Aizen D, Sarfstein R, Bruchim I, Weinstein D, Laron Z, Werner H. Proliferative and signaling activities of insulin analogues in endometrial cancer cells. Mol Cell Endocrinol. Elsevier; 2015;406: 27–39. doi: 10.1016/j.mce.2015.02.011 - DOI - PubMed
-
- Solomon Zemler R, Weingarten G, Sarfstein R, Laron Z, Werner H, Wertheimer E. Insulin analogues display atypical differentiative activities in skin keratinocytes. Arch Physiol Biochem. Taylor & Francis; 2015;121: 32–39. doi: 10.3109/13813455.2014.1001856 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

