Characterisation of insulin analogues therapeutically available to patients
- PMID: 29596514
- PMCID: PMC5875863
- DOI: 10.1371/journal.pone.0195010
Characterisation of insulin analogues therapeutically available to patients
Abstract
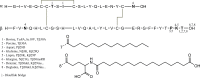
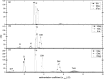
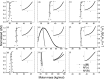
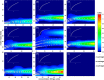
The structure and function of clinical dosage insulin and its analogues were assessed. This included 'native insulins' (human recombinant, bovine, porcine), 'fast-acting analogues' (aspart, glulisine, lispro) and 'slow-acting analogues' (glargine, detemir, degludec). Analytical ultracentrifugation, both sedimentation velocity and equilibrium experiments, were employed to yield distributions of both molar mass and sedimentation coefficient of all nine insulins. Size exclusion chromatography, coupled to multi-angle light scattering, was also used to explore the function of these analogues. On ultracentrifugation analysis, the insulins under investigation were found to be in numerous conformational states, however the majority of insulins were present in a primarily hexameric conformation. This was true for all native insulins and two fast-acting analogues. However, glargine was present as a dimer, detemir was a multi-hexameric system, degludec was a dodecamer (di-hexamer) and glulisine was present as a dimer-hexamer-dihexamer system. However, size-exclusion chromatography showed that the two hexameric fast-acting analogues (aspart and lispro) dissociated into monomers and dimers due to the lack of zinc in the mobile phase. This comprehensive study is the first time all nine insulins have been characterised in this way, the first time that insulin detemir have been studied using analytical ultracentrifugation and the first time that insulins aspart and glulisine have been studied using sedimentation equilibrium. The structure and function of these clinically administered insulins is of critical importance and this research adds novel data to an otherwise complex functional physiological protein.
Conflict of interest statement
Figures

References
-
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. Rockefeller University Press; 2008;183: 1235–1242. doi: 10.1083/jcb.200809113 - DOI - PMC - PubMed
-
- Adams GG, Alzahrani Q, Jiwani SI, Meal A, Morgan PS, Coffey F, et al. Glargine and degludec: Solution behaviour of higher dose synthetic insulins. Sci Rep. Nature Publishing Group; 2017;7: 7287 doi: 10.1038/s41598-017-06642-w - DOI - PMC - PubMed
-
- Aizen D, Sarfstein R, Bruchim I, Weinstein D, Laron Z, Werner H. Proliferative and signaling activities of insulin analogues in endometrial cancer cells. Mol Cell Endocrinol. Elsevier; 2015;406: 27–39. doi: 10.1016/j.mce.2015.02.011 - DOI - PubMed
-
- Solomon Zemler R, Weingarten G, Sarfstein R, Laron Z, Werner H, Wertheimer E. Insulin analogues display atypical differentiative activities in skin keratinocytes. Arch Physiol Biochem. Taylor & Francis; 2015;121: 32–39. doi: 10.3109/13813455.2014.1001856 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

