Carcinoma associated fibroblasts (CAFs) promote breast cancer motility by suppressing mammalian Diaphanous-related formin-2 (mDia2)
- PMID: 29596520
- PMCID: PMC5875872
- DOI: 10.1371/journal.pone.0195278
Carcinoma associated fibroblasts (CAFs) promote breast cancer motility by suppressing mammalian Diaphanous-related formin-2 (mDia2)
Abstract
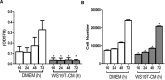
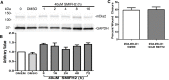
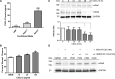
The tumor microenvironment (TME) promotes tumor cell invasion and metastasis. An important step in the shift to a pro-cancerous microenvironment is the transformation of normal stromal fibroblasts to carcinoma-associated fibroblasts (CAFs). CAFs are present in a majority of solid tumors and can directly promote tumor cell motility via cytokine, chemokine and growth factor secretion into the TME. The exact effects that the TME has upon cytoskeletal regulation in motile tumor cells remain enigmatic. The conserved formin family of cytoskeleton regulating proteins plays an essential role in the assembly and/or bundling of unbranched actin filaments. Mammalian Diaphanous-related formin 2 (mDia2/DIAPH3/Drf3/Dia) assembles a dynamic F-actin cytoskeleton that underlies tumor cell migration and invasion. We therefore sought to understand whether CAF-derived chemokines impact breast tumor cell motility through modification of the formin-assembled F-actin cytoskeleton. In MDA-MB-231 cells, conditioned media (CM) from WS19T CAFs, a human breast tumor-adjacent CAF line, significantly and robustly increased wound closure and invasion relative to normal human mammary fibroblast (HMF)-CM. WS19T-CM also promoted proteasome-mediated mDia2 degradation in MDA-MB-231 cells relative to control HMF-CM and WS21T CAF-CM, a breast CAF cell line that failed to promote robust MDA-MB-231 migration. Cytokine array analysis of CM identified up-regulated secreted factors in WS19T relative to control WS21T CM. We identified CXCL12 as a CM factor influencing loss of mDia2 protein while increasing MDA-MB-231 cell migration. Our data suggest a mechanism whereby CAFs promote tumor cell migration and invasion through CXCL12 secretion to regulate the mDia2-directed cytoskeleton in breast tumor cells.
Conflict of interest statement
Figures







Similar articles
-
mDia2 and CXCL12/CXCR4 chemokine signaling intersect to drive tumor cell amoeboid morphological transitions.Biochem Biophys Res Commun. 2017 Mar 4;484(2):255-261. doi: 10.1016/j.bbrc.2017.01.087. Epub 2017 Jan 21. Biochem Biophys Res Commun. 2017. PMID: 28115158 Free PMC article.
-
Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-α and the NF-κB pathway.Stem Cell Res Ther. 2015 May 1;6(1):87. doi: 10.1186/s13287-015-0080-7. Stem Cell Res Ther. 2015. PMID: 25928089 Free PMC article.
-
Fibroblast growth factor-2, derived from cancer-associated fibroblasts, stimulates growth and progression of human breast cancer cells via FGFR1 signaling.Mol Carcinog. 2020 Sep;59(9):1028-1040. doi: 10.1002/mc.23233. Epub 2020 Jun 18. Mol Carcinog. 2020. PMID: 32557854
-
Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways.J Exp Clin Cancer Res. 2020 Jun 16;39(1):112. doi: 10.1186/s13046-020-01611-0. J Exp Clin Cancer Res. 2020. PMID: 32546182 Free PMC article. Review.
-
Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment.Cancer Metastasis Rev. 2018 Dec;37(4):577-597. doi: 10.1007/s10555-018-9768-3. Cancer Metastasis Rev. 2018. PMID: 30465162 Review.
Cited by
-
Cancer-Associated Fibroblasts: Epigenetic Regulation and Therapeutic Intervention in Breast Cancer.Cancers (Basel). 2020 Oct 13;12(10):2949. doi: 10.3390/cancers12102949. Cancers (Basel). 2020. PMID: 33066013 Free PMC article. Review.
-
The Role of Cancer-Associated Fibroblasts in Tumor Progression.Cancers (Basel). 2021 Mar 19;13(6):1399. doi: 10.3390/cancers13061399. Cancers (Basel). 2021. PMID: 33808627 Free PMC article. Review.
-
Loss of DIAPH3, a Formin Family Protein, Leads to Cytokinetic Failure Only under High Temperature Conditions in Mouse FM3A Cells.Int J Mol Sci. 2020 Nov 11;21(22):8493. doi: 10.3390/ijms21228493. Int J Mol Sci. 2020. PMID: 33187357 Free PMC article.
-
Transparent 3-Layered Bacterial Nanocellulose as a Multicompartment and Biomimetic Scaffold for Co-Culturing Cells.J Funct Biomater. 2025 Jun 3;16(6):208. doi: 10.3390/jfb16060208. J Funct Biomater. 2025. PMID: 40558895 Free PMC article.
-
Cancer-associated fibroblasts in breast cancer: Challenges and opportunities.Cancer Commun (Lond). 2022 May;42(5):401-434. doi: 10.1002/cac2.12291. Epub 2022 Apr 28. Cancer Commun (Lond). 2022. PMID: 35481621 Free PMC article. Review.
References
-
- Sporn MB. The war on cancer. Lancet. 1996;347(9012):1377–81. Epub 1996/05/18. . - PubMed
-
- Witz IP, Levy-Nissenbaum O. The tumor microenvironment in the post-PAGET era. Cancer Lett. 2006;242(1):1–10. Epub 2006/01/18. doi: 10.1016/j.canlet.2005.12.005 . - DOI - PubMed
-
- Wang W, Li Q, Yamada T, Matsumoto K, Matsumoto I, Oda M, et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15(21):6630–8. Epub 2009/10/22. doi: 10.1158/1078-0432.ccr-09-1001 . - DOI - PubMed
-
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science (New York, NY). 2004;303(5659):848–51. Epub 2004/02/07. doi: 10.1126/science.1090922 . - DOI - PubMed
-
- Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, et al. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124(4):853–61. doi: 10.1002/ijc.24040 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

