Hostile intruder: Toxoplasma holds host organelles captive
- PMID: 29596535
- PMCID: PMC5875880
- DOI: 10.1371/journal.ppat.1006893
Hostile intruder: Toxoplasma holds host organelles captive
Erratum in
-
Correction: Hostile intruder: Toxoplasma holds host organelles captive.PLoS Pathog. 2018 Apr 24;14(4):e1007018. doi: 10.1371/journal.ppat.1007018. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29689101 Free PMC article.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
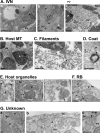
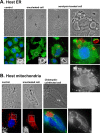
References
-
- Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J Cell Sci. 1995;108 (Pt 4): 1669–1677. - PubMed
-
- Magno RC, Lemgruber L, Vommaro RC, De Souza W, Attias M. Intravacuolar network may act as a mechanical support for Toxoplasma gondii inside the parasitophorous vacuole. Microsc Res Tech. 2005;67: 45–52. doi: 10.1002/jemt.20182 - DOI - PubMed
-
- De Souza W, Attias M. New views of the Toxoplasma gondii parasitophorous vacuole as revealed by Helium Ion Microscopy (HIM). J Struct Biol. 2015;191: 76–85. doi: 10.1016/j.jsb.2015.05.003 - DOI - PubMed
-
- Mercier C, Dubremetz J-F, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw M-F. Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol Biol Cell. 2002;13: 2397–2409. doi: 10.1091/mbc.E02-01-0021 - DOI - PMC - PubMed
-
- Caffaro CE, Boothroyd JC. Evidence for host cells as the major contributor of lipids in the intravacuolar network of Toxoplasma-infected cells. Eukaryotic Cell. 2011;10: 1095–1099. doi: 10.1128/EC.00002-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

