A structurally conserved motif in γ-herpesvirus uracil-DNA glycosylases elicits duplex nucleotide-flipping
- PMID: 29596604
- PMCID: PMC5934625
- DOI: 10.1093/nar/gky217
A structurally conserved motif in γ-herpesvirus uracil-DNA glycosylases elicits duplex nucleotide-flipping
Abstract
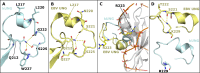
Efficient γ-herpesvirus lytic phase replication requires a virally encoded UNG-type uracil-DNA glycosylase as a structural element of the viral replisome. Uniquely, γ-herpesvirus UNGs carry a seven or eight residue insertion of variable sequence in the otherwise highly conserved minor-groove DNA binding loop. In Epstein-Barr Virus [HHV-4] UNG, this motif forms a disc-shaped loop structure of unclear significance. To ascertain the biological role of the loop insertion, we determined the crystal structure of Kaposi's sarcoma-associated herpesvirus [HHV-8] UNG (kUNG) in its product complex with a uracil-containing dsDNA, as well as two structures of kUNG in its apo state. We find the disc-like conformation is conserved, but only when the kUNG DNA-binding cleft is occupied. Surprisingly, kUNG uses this structure to flip the orphaned partner base of the substrate deoxyuridine out of the DNA duplex while retaining canonical UNG deoxyuridine-flipping and catalysis. The orphan base is stably posed in the DNA major groove which, due to DNA backbone manipulation by kUNG, is more open than in other UNG-dsDNA structures. Mutagenesis suggests a model in which the kUNG loop is pinned outside the DNA-binding cleft until DNA docking promotes rigid structuring of the loop and duplex nucleotide flipping, a novel observation for UNGs.
Figures




Similar articles
-
New insights on the role of the gamma-herpesvirus uracil-DNA glycosylase leucine loop revealed by the structure of the Epstein-Barr virus enzyme in complex with an inhibitor protein.J Mol Biol. 2007 Feb 9;366(1):117-31. doi: 10.1016/j.jmb.2006.11.007. Epub 2006 Nov 7. J Mol Biol. 2007. PMID: 17157317
-
Structure determination of uracil-DNA N-glycosylase from Deinococcus radiodurans in complex with DNA.Acta Crystallogr D Biol Crystallogr. 2015 Oct;71(Pt 10):2137-49. doi: 10.1107/S1399004715014157. Epub 2015 Sep 30. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26457437
-
Herpes simplex virus 1 evades APOBEC1-mediated immunity via its uracil-DNA glycosylase in mice.Nat Microbiol. 2025 Jul;10(7):1758-1774. doi: 10.1038/s41564-025-02026-3. Epub 2025 Jun 3. Nat Microbiol. 2025. PMID: 40461648
-
Lumbar sympathectomy versus prostanoids for critical limb ischaemia due to non-reconstructable peripheral arterial disease.Cochrane Database Syst Rev. 2018 Apr 16;4(4):CD009366. doi: 10.1002/14651858.CD009366.pub2. Cochrane Database Syst Rev. 2018. PMID: 29658630 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Human DNA tumor viruses evade uracil-mediated antiviral immunity.PLoS Pathog. 2023 Mar 30;19(3):e1011252. doi: 10.1371/journal.ppat.1011252. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36996040 Free PMC article. No abstract available.
-
Self-Repairing Herpesvirus Saimiri Deletion Variants.Viruses. 2022 Jul 13;14(7):1525. doi: 10.3390/v14071525. Viruses. 2022. PMID: 35891505 Free PMC article.
-
Sequencing of Kaposi's Sarcoma Herpesvirus (KSHV) genomes from persons of diverse ethnicities and provenances with KSHV-associated diseases demonstrate multiple infections, novel polymorphisms, and low intra-host variance.PLoS Pathog. 2024 Jul 15;20(7):e1012338. doi: 10.1371/journal.ppat.1012338. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39008527 Free PMC article.
-
Mechanisms of DNA Damage Recognition by UDG and PARP1 in the Nucleosome.Biomolecules. 2025 Apr 30;15(5):649. doi: 10.3390/biom15050649. Biomolecules. 2025. PMID: 40427542 Free PMC article.
-
The Essential Co-Option of Uracil-DNA Glycosylases by Herpesviruses Invites Novel Antiviral Design.Microorganisms. 2020 Mar 24;8(3):461. doi: 10.3390/microorganisms8030461. Microorganisms. 2020. PMID: 32214054 Free PMC article. Review.
References
-
- Cesarman E., Chang Y., Moore P.S., Said J.W., Knowles D.M.. Kaposi's sarcoma–associated herpesvirus-like dna sequences in aids-related body-cavity–based lymphomas e. N. Engl. J. Med. 1995; 332:1186–1191. - PubMed
-
- Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d’Agay M.F., Clauvel J.P., Raphael M., Degos L.. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995; 86:1276–1280. - PubMed
-
- Arvanitakis L., Mesri E.A., Nador R.G., Said J.W., Asch A.S., Knowles D.M., Cesarman E.. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996; 88:2648–2654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

