Stereospecific Ring Contraction of Bromocycloheptenes through Dyotropic Rearrangements via Nonclassical Carbocation-Anion Pairs
- PMID: 29596748
- PMCID: PMC5909176
- DOI: 10.1021/jacs.8b00821
Stereospecific Ring Contraction of Bromocycloheptenes through Dyotropic Rearrangements via Nonclassical Carbocation-Anion Pairs
Abstract
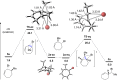
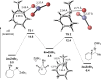
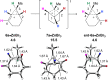
Experimental and theoretical evidence is reported for a rare type I dyotropic rearrangement involving a [1,2]-alkene shift, leading to the regio- and stereospecific ring contraction of bromocycloheptenes. This reaction occurs under mild conditions, with or without a Lewis acid catalyst. DFT calculations show that the reaction proceeds through a nonclassical carbocation-anion pair, which is crucial for the low activation barrier and enantiospecificity. The chiral cyclopropylcarbinyl cation may be a transition state or an intermediate, depending on the reaction conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
-
Desymmetrization of meso-dibromocycloalkenes with organolithium reagents: unpublished manuscript.
-
-
-
For reviews of Cu-catalyzed AAS, see:
- Geurts K.; Fletcher S. P.; van Zijl A. W.; Minnaard A. J.; Feringa B. L. Pure Appl. Chem. 2008, 80, 1025–1037. 10.1351/pac200880051025. - DOI
- Harutyunyan S. R.; den Hartog T.; Geurts K.; Minnaard A. J.; Feringa B. L. Chem. Rev. 2008, 108, 2824–2852. 10.1021/cr068424k. - DOI - PubMed
- Alexakis A.; Bäckvall J. E.; Krause N.; Pàmies O.; Diéguez M. Chem. Rev. 2008, 108, 2796.10.1021/cr0683515. - DOI - PubMed
- Langlois J.-B.; Alexakis A. In Transition Metal Catalyzed Allylic Substitution in Organic Synthesis; Kazmaier U., Ed.; Springer-Verlag: Berlin, 2012; pp 235–268.
- Hornillos V.; Gualtierotti J.-B.; Feringa B. L. Top. Organomet. Chem. 2016, 58, 1–39. 10.1007/3418_2015_165. - DOI
- Baslé O.; Denicourt-Nowicki A.; Crévisy C.; Mauduit M. In Copper-Catalyzed Asymmetric Synthesis; Alexakis A., Krause N., Woodward S., Eds.; Wiley-VCH: Weinheim, 2014; pp 85–126.
-
-
-
For selected examples of asymmetric allylic substitution with organolithium reagents, see:
- Pérez M.; Fañanás-Mastral M.; Bos P. H.; Rudolph A.; Harutyunyan S. R.; Feringa B. L. Nat. Chem. 2011, 3, 377.10.1038/nchem.1009. - DOI - PubMed
- Fañanás-Mastral M.; Pérez M.; Bos P. H.; Rudolph A.; Harutyunyan S. R.; Feringa B. L. Angew. Chem., Int. Ed. 2012, 51, 1922.10.1002/anie.201107840. - DOI - PubMed
- Guduguntla S.; Gualtierotti J.-B.; Goh S. S.; Feringa B. L. ACS Catal. 2016, 6, 6591.10.1021/acscatal.6b01681. - DOI
- Hornillos V.; Guduguntla S.; Fañanás-Mastral M.; Pérez M.; Bos P. H.; Rudolph A.; Harutyunyan S. R.; Feringa B. L. Nat. Protoc. 2017, 12, 493.10.1038/nprot.2016.179. - DOI - PubMed
-
-
-
CCDC 1571256 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
-
-
-
For selected examples of crystalline sponge X-ray crystallography, see:
- Inokuma Y.; Yoshioka S.; Ariyoshi J.; Arai T.; Hitora Y.; Takada K.; Matsunaga S.; Rissanen K.; Fujita M. Nature 2013, 495, 461.10.1038/nature11990. - DOI - PubMed
- Inokuma Y.; Yoshioka S.; Ariyoshi J.; Arai T.; Fujita M. Nat. Protoc. 2014, 9, 246.10.1038/nprot.2014.007. - DOI - PubMed
- Hoshino M.; Khutia A.; Xing H.; Inokuma Y.; Fujita M. IUCrJ 2016, 3, 139.10.1107/S2052252515024379. - DOI - PMC - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

