Some Surprising Implications of NMR-directed Simulations of Substrate Recognition and Binding by Cytochrome P450cam (CYP101A1)
- PMID: 29596916
- PMCID: PMC5916322
- DOI: 10.1016/j.jmb.2018.03.014
Some Surprising Implications of NMR-directed Simulations of Substrate Recognition and Binding by Cytochrome P450cam (CYP101A1)
Abstract
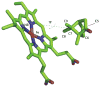
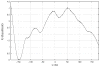
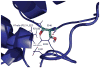
Cytochrome P450cam (CYP101A1) catalyzes the stereospecific 5-exo hydroxylation of d-camphor by molecular oxygen. Previously, residual dipolar couplings measured for backbone amide 1H-15N correlations in both substrate-free and bound forms of CYP101A1 were used as restraints in soft annealing molecular dynamic simulations in order to identify average conformations of the enzyme with and without substrate bound. Multiple substrate-dependent conformational changes remote from the enzyme active site were identified, and site-directed mutagenesis and activity assays confirmed the importance of these changes in substrate recognition. The current work makes use of perturbation response scanning (PRS) and umbrella sampling molecular dynamic of the residual dipolar coupling-derived CYP101A1 structures to probe the roles of remote structural features in enforcing the regio- and stereospecific nature of the hydroxylation reaction catalyzed by CYP101A1. An improper dihedral angle Ψ was defined and used to maintain substrate orientation in the CYP101A1 active site, and it was observed that different values of Ψ result in different PRS response maps. Umbrella sampling methods show that the free energy of the system is sensitive to Ψ, and bound substrate forms an important mechanical link in the transmission of mechanical coupling through the enzyme structure. Finally, a qualitative approach to interpreting PRS maps in terms of the roles of secondary structural features is proposed.
Keywords: anisotropic network model; cytochrome P450 stereospecificity; perturbation response scanning; residual dipolar couplings; solution conformational ensembles.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures










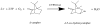
Similar articles
-
What Your Crystal Structure Will Not Tell You about Enzyme Function.Acc Chem Res. 2019 May 21;52(5):1409-1418. doi: 10.1021/acs.accounts.9b00066. Epub 2019 Apr 29. Acc Chem Res. 2019. PMID: 31034199 Free PMC article.
-
Hydroxylation Regiochemistry Is Robust to Active Site Mutations in Cytochrome P450cam (CYP101A1).Biochemistry. 2022 Sep 6;61(17):1790-1800. doi: 10.1021/acs.biochem.2c00233. Epub 2022 Aug 12. Biochemistry. 2022. PMID: 35960510 Free PMC article.
-
Experimentally restrained molecular dynamics simulations for characterizing the open states of cytochrome P450cam.Biochemistry. 2011 Mar 15;50(10):1664-71. doi: 10.1021/bi101820d. Epub 2011 Feb 8. Biochemistry. 2011. PMID: 21265500 Free PMC article.
-
Differential behavior of the sub-sites of cytochrome 450 active site in binding of substrates, and products (implications for coupling/uncoupling).Biochim Biophys Acta. 2007 Mar;1770(3):360-75. doi: 10.1016/j.bbagen.2006.09.018. Epub 2006 Oct 5. Biochim Biophys Acta. 2007. PMID: 17134838 Review.
-
Cytochrome P450.Curr Opin Struct Biol. 1995 Dec;5(6):767-74. doi: 10.1016/0959-440x(95)80009-3. Curr Opin Struct Biol. 1995. PMID: 8749364 Review.
Cited by
-
Entropic contribution to enhanced thermal stability in the thermostable P450 CYP119.Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):E10049-E10058. doi: 10.1073/pnas.1807473115. Epub 2018 Oct 8. Proc Natl Acad Sci U S A. 2018. PMID: 30297413 Free PMC article.
-
Ligand and Redox Partner Binding Generates a New Conformational State in Cytochrome P450cam (CYP101A1).J Am Chem Soc. 2019 Feb 13;141(6):2678-2683. doi: 10.1021/jacs.8b13079. Epub 2019 Jan 31. J Am Chem Soc. 2019. PMID: 30672701 Free PMC article.
-
What Your Crystal Structure Will Not Tell You about Enzyme Function.Acc Chem Res. 2019 May 21;52(5):1409-1418. doi: 10.1021/acs.accounts.9b00066. Epub 2019 Apr 29. Acc Chem Res. 2019. PMID: 31034199 Free PMC article.
-
A new approach to understanding structure-function relationships in cytochromes P450 by targeting terpene metabolism in the wild.J Inorg Biochem. 2018 Nov;188:96-101. doi: 10.1016/j.jinorgbio.2018.08.006. Epub 2018 Aug 6. J Inorg Biochem. 2018. PMID: 30170307 Free PMC article.
-
Hydroxylation Regiochemistry Is Robust to Active Site Mutations in Cytochrome P450cam (CYP101A1).Biochemistry. 2022 Sep 6;61(17):1790-1800. doi: 10.1021/acs.biochem.2c00233. Epub 2022 Aug 12. Biochemistry. 2022. PMID: 35960510 Free PMC article.
References
-
- Prestegard JH, Bougault CM, Kishore AI. Residual dipolar couplings in structure determination of biomolecules. Chemical Reviews. 2004;104:3519–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

