Characterization of Hit Compounds Identified from High-throughput Screening for their Effect on Blood-brain Barrier Integrity and Amyloid-β Clearance: In Vitro and In Vivo Studies
- PMID: 29596966
- PMCID: PMC5924725
- DOI: 10.1016/j.neuroscience.2018.03.028
Characterization of Hit Compounds Identified from High-throughput Screening for their Effect on Blood-brain Barrier Integrity and Amyloid-β Clearance: In Vitro and In Vivo Studies
Abstract
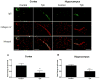
In Alzheimer's disease (AD) the blood-brain barrier (BBB) is compromised, thus therapeutic targeting of the BBB to enhance its integrity and function could be a unique approach to treat, slow or hold the progression of AD. Recently, we have developed an in vitro high-throughput screening assay to screen for compounds that increase the integrity of a cell-based BBB model. Results from primary screen identified multiple hit compounds that enhanced the monolayer integrity. Herein, further characterization of selected hit compounds, namely 8-bromoguanosine cyclic monophosphate, JW74, 1,10-phenanthroline monohydrate, SB216763 and α-tocopherol was performed. Compounds were subjected to concentration-dependent studies to determine their EC50 and potency to enhance the cell-based model integrity by the Lucifer Yellow permeability and amyloid-beta (Aβ) transport across the monolayer. The compounds demonstrated different EC50s to enhance the monolayer integrity ranging from 0.4 to 12.8 µM, and different effect on enhancing Aβ transport with highest transport observed for α-tocopherol (2.2-fold increase). Such effects were associated with increased levels of tight junction proteins such as claudin-5 and/or ZO-1, and Aβ major transport proteins LRP1 and P-glycoprotein. In vivo studies for α-tocopherol were performed in AD mouse model; consistent with the in vitro results α-tocopherol significantly increased BBB integrity measured by IgG extravasation, and reduced brain Aβ levels. In conclusion, findings support our developed cell-based BBB model as a functional predictive in vivo tool to select hit compounds, and suggest that enhancing BBB tightness and function has the potential to reduce Aβ pathology associated with AD.
Keywords: IgG extravasation; amyloid-β clearance; blood–brain barrier; brain endothelial cells; high-throughput screening; α-tocopherol.
Copyright © 2018 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

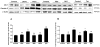
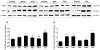



Similar articles
-
Blood-Brain Barrier Disruption Increases Amyloid-Related Pathology in TgSwDI Mice.Int J Mol Sci. 2021 Jan 27;22(3):1231. doi: 10.3390/ijms22031231. Int J Mol Sci. 2021. PMID: 33513818 Free PMC article.
-
Inhibition of ADAM10 promotes the clearance of Aβ across the BBB by reducing LRP1 ectodomain shedding.Fluids Barriers CNS. 2016 Aug 8;13(1):14. doi: 10.1186/s12987-016-0038-x. Fluids Barriers CNS. 2016. PMID: 27503326 Free PMC article.
-
Aβ₁₋₄₂-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca²⁺-calcineurin signaling.J Neurosci. 2012 Jun 27;32(26):8845-54. doi: 10.1523/JNEUROSCI.6102-11.2012. J Neurosci. 2012. PMID: 22745485 Free PMC article.
-
P-glycoprotein: a role in the export of amyloid-β in Alzheimer's disease?FEBS J. 2020 Feb;287(4):612-625. doi: 10.1111/febs.15148. Epub 2019 Dec 9. FEBS J. 2020. PMID: 31750987 Review.
-
Drug access to the central nervous system in Alzheimer's disease: preclinical and clinical insights.Pharm Res. 2015 Mar;32(3):819-39. doi: 10.1007/s11095-014-1522-0. Epub 2014 Oct 16. Pharm Res. 2015. PMID: 25319097 Review.
Cited by
-
Association of PTHrP levels in CSF with Alzheimer's disease biomarkers.Clin Mass Spectrom. 2018 Oct 9;14 Pt B:124-129. doi: 10.1016/j.clinms.2018.10.001. eCollection 2019 Nov. Clin Mass Spectrom. 2018. PMID: 34917769 Free PMC article.
-
Blood-Brain Barrier Disruption Increases Amyloid-Related Pathology in TgSwDI Mice.Int J Mol Sci. 2021 Jan 27;22(3):1231. doi: 10.3390/ijms22031231. Int J Mol Sci. 2021. PMID: 33513818 Free PMC article.
-
Multi-faceted therapeutic strategy for treatment of Alzheimer's disease by concurrent administration of etodolac and α-tocopherol.Neurobiol Dis. 2019 May;125:123-134. doi: 10.1016/j.nbd.2019.01.020. Epub 2019 Jan 30. Neurobiol Dis. 2019. PMID: 30710675 Free PMC article.
-
Approaches for Increasing Cerebral Efflux of Amyloid-β in Experimental Systems.J Alzheimers Dis. 2024;100(2):379-411. doi: 10.3233/JAD-240212. J Alzheimers Dis. 2024. PMID: 38875041 Free PMC article. Review.
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

