Identification of Leaf Promoters for Use in Transgenic Wheat
- PMID: 29597282
- PMCID: PMC6027260
- DOI: 10.3390/plants7020027
Identification of Leaf Promoters for Use in Transgenic Wheat
Abstract
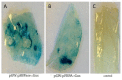
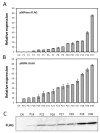
Wheat yields have plateaued in recent years and given the growing global population there is a pressing need to develop higher yielding varieties to meet future demand. Genetic manipulation of photosynthesis in elite wheat varieties offers the opportunity to significantly increase yields. However, the absence of a well-defined molecular tool-box of promoters to manipulate leaf processes in wheat hinders advancements in this area. Two promoters, one driving the expression of sedoheptulose-1,7-bisphosphatase (SBPase) and the other fructose-1,6-bisphosphate aldolase (FBPA) from Brachypodium distachyon were identified and cloned into a vector in front of the GUS reporter gene. Both promoters were shown to be functionally active in wheat in both transient assays and in stably transformed wheat plants. Analysis of the stable transformants of wheat (cv. Cadenza) showed that both promoters controlled gus expression throughout leaf development as well as in other green tissues. The availability of these promoters provides new tools for the expression of genes in transgenic wheat leaves and also paves the way for multigene manipulation of photosynthesis to improve yields.
Keywords: photosynthesis; promoter; reporter gene; tissue specific; wheat; yield.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Braun H.J., Atlin G., Payne T. Multi-location testing as a tool to identify plant response to global climate change. In: Reynolds M.P., editor. Climate Change and Crop Production. CABI; Surrey, UK: 2010. pp. 115–138. (Climate Change Series).
-
- Godfray H.C.J. The challenge of feeding 9–10 billion people equitably and sustainably. J. Agric. Sci. 2014;152:S2–S8. doi: 10.1017/S0021859613000774. - DOI
-
- RSOL . Royal Society of London, Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture. Royal Society; London, UK: 2009.
LinkOut - more resources
Full Text Sources
Other Literature Sources

