Polymer-Mediated Delivery of siRNAs to Hepatocellular Carcinoma: Variables Affecting Specificity and Effectiveness
- PMID: 29597300
- PMCID: PMC6017305
- DOI: 10.3390/molecules23040777
Polymer-Mediated Delivery of siRNAs to Hepatocellular Carcinoma: Variables Affecting Specificity and Effectiveness
Abstract
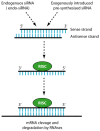
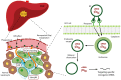
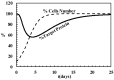
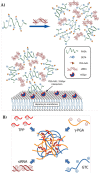
Despite the advances in anticancer therapies, their effectiveness for many human tumors is still far from being optimal. Significant improvements in treatment efficacy can come from the enhancement of drug specificity. This goal may be achieved by combining the use of therapeutic molecules with tumor specific effects and delivery carriers with tumor targeting ability. In this regard, nucleic acid-based drug (NABD) and particularly small interfering RNAs (siRNAs), are attractive molecules due to the possibility to be engineered to target specific tumor genes. On the other hand, polymeric-based delivery systems are emerging as versatile carriers to generate tumor-targeted delivery systems. Here we will focus on the most recent findings in the selection of siRNA/polymeric targeted delivery systems for hepatocellular carcinoma (HCC), a human tumor for which currently available therapeutic approaches are poorly effective. In addition, we will discuss the most attracting and, in our opinion, promising siRNA-polymer combinations for HCC in relation to the biological features of HCC tissue. Attention will be also put on the mathematical description of the mechanisms ruling siRNA-carrier delivery, this being an important aspect to improve effectiveness reducing the experimental work.
Keywords: HCC; optimized drug delivery; siRNA.
Conflict of interest statement
The authors declare no conflict of interest. “The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results”.
Figures


References
-
- Agostini F., Dapas B., Farra R., Grassi M., Racchi G., Klingel K., Kandolf R., Heidenreich O., Mercatahnti A., Rainaldi G., et al. Potential applications of small interfering RNAs in the cardiovascular field. Drug Future. 2006;31:513–525. doi: 10.1358/dof.2006.031.06.995893. - DOI
-
- Grassi M., Cavallaro G., Scirè S., Scaggiante B., Daps B., Farra R., Baiz D., Giansante C., Guarnieri G., Perin D., et al. Current Strategies to Improve the Efficacy and the Delivery of Nucleic Acid Based Drugs. Curr. Signal Transduct. Ther. 2010;5:92–120. doi: 10.2174/157436210791112163. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

