Establishment of human pluripotent stem cell-derived pancreatic β-like cells in the mouse pancreas
- PMID: 29599125
- PMCID: PMC5899428
- DOI: 10.1073/pnas.1702059115
Establishment of human pluripotent stem cell-derived pancreatic β-like cells in the mouse pancreas
Abstract
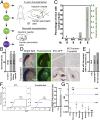
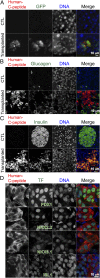
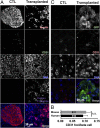
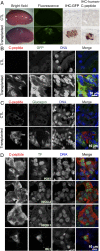
Type 1 diabetes is characterized by autoimmune destruction of β cells located in pancreatic islets. However, tractable in vivo models of human pancreatic β cells have been limited. Here, we generated xenogeneic human pancreatic β-like cells in the mouse pancreas by orthotopic transplantation of stem cell-derived β (SC-β) cells into the pancreas of neonatal mice. The engrafted β-like cells expressed β cell transcription factors and markers associated with functional maturity. Engrafted human cells recruited mouse endothelial cells, suggesting functional integration. Human insulin was detected in the blood circulation of transplanted mice for months after transplantation and increased upon glucose stimulation. In addition to β-like cells, human cells expressing markers for other endocrine pancreas cell types, acinar cells, and pancreatic ductal cells were identified in the pancreata of transplanted mice, indicating that this approach allows studying other human pancreatic cell types in the mouse pancreas. Our results demonstrate that orthotopic transplantation of human SC-β cells into neonatal mice is an experimental platform that allows the generation of mice with human pancreatic β-like cells in the endogenous niche.
Keywords: beta cells; diabetes; human pluripotent stem cells; humanized mice.
Conflict of interest statement
Conflict of interest statement: R.J. is a cofounder and advisor of Fate Therapeutics, Fulcrum Therapeutics, and Omega Therapeutics. D.A.M. is the founder of Semma Therapeutics.
Figures
Similar articles
-
In vitro generation of pancreatic β-cells for diabetes treatment. I. β-like cells derived from human pluripotent stem cells.Folia Histochem Cytobiol. 2019;57(1):1-14. doi: 10.5603/FHC.a2019.0001. Epub 2019 Mar 14. Folia Histochem Cytobiol. 2019. PMID: 30869153 Review.
-
An arduous journey from human pluripotent stem cells to functional pancreatic β cells.Diabetes Obes Metab. 2018 Jan;20(1):3-13. doi: 10.1111/dom.12996. Epub 2017 Jun 7. Diabetes Obes Metab. 2018. PMID: 28474496 Review.
-
Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo.Sci Rep. 2016 Oct 12;6:35145. doi: 10.1038/srep35145. Sci Rep. 2016. PMID: 27731367 Free PMC article.
-
In Vitro Differentiation of Pluripotent Stem Cells into Functional β Islets Under 2D and 3D Culture Conditions and In Vivo Preclinical Validation of 3D Islets.Methods Mol Biol. 2016;1341:257-84. doi: 10.1007/7651_2015_230. Methods Mol Biol. 2016. PMID: 25783769
-
Long-Term Culture of Self-renewing Pancreatic Progenitors Derived from Human Pluripotent Stem Cells.Stem Cell Reports. 2017 Jun 6;8(6):1675-1688. doi: 10.1016/j.stemcr.2017.05.019. Stem Cell Reports. 2017. PMID: 28591650 Free PMC article.
Cited by
-
From iPSCs to Pancreatic β Cells: Unveiling Molecular Pathways and Enhancements with Vitamin C and Retinoic Acid in Diabetes Research.Int J Mol Sci. 2024 Sep 6;25(17):9654. doi: 10.3390/ijms25179654. Int J Mol Sci. 2024. PMID: 39273600 Free PMC article.
-
Gastrin producing syngeneic mesenchymal stem cells protect non-obese diabetic mice from type 1 diabetes.Autoimmunity. 2022 Mar;55(2):95-108. doi: 10.1080/08916934.2021.2012165. Epub 2021 Dec 9. Autoimmunity. 2022. PMID: 34882054 Free PMC article.
-
Multi-lineage Human iPSC-Derived Platforms for Disease Modeling and Drug Discovery.Cell Stem Cell. 2020 Mar 5;26(3):309-329. doi: 10.1016/j.stem.2020.02.011. Cell Stem Cell. 2020. PMID: 32142662 Free PMC article. Review.
-
Metabolic Changes during In Vivo Maturation of PSC-Derived Skeletal Myogenic Progenitors.Cells. 2023 Dec 29;13(1):76. doi: 10.3390/cells13010076. Cells. 2023. PMID: 38201280 Free PMC article.
-
Mitigating Antagonism between Transcription and Proliferation Allows Near-Deterministic Cellular Reprogramming.Cell Stem Cell. 2019 Oct 3;25(4):486-500.e9. doi: 10.1016/j.stem.2019.08.005. Epub 2019 Sep 12. Cell Stem Cell. 2019. PMID: 31523028 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

