CaMKII (Ca2+/Calmodulin-Dependent Kinase II) in Mitochondria of Smooth Muscle Cells Controls Mitochondrial Mobility, Migration, and Neointima Formation
- PMID: 29599132
- PMCID: PMC5970052
- DOI: 10.1161/ATVBAHA.118.310951
CaMKII (Ca2+/Calmodulin-Dependent Kinase II) in Mitochondria of Smooth Muscle Cells Controls Mitochondrial Mobility, Migration, and Neointima Formation
Abstract
Objective: The main objective of this study is to define the mechanisms by which mitochondria control vascular smooth muscle cell (VSMC) migration and impact neointimal hyperplasia.
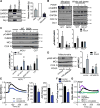
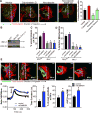
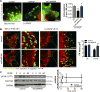
Approach and results: The multifunctional CaMKII (Ca2+/calmodulin-dependent kinase II) in the mitochondrial matrix of VSMC drove a feed-forward circuit with the mitochondrial Ca2+ uniporter (MCU) to promote matrix Ca2+ influx. MCU was necessary for the activation of mitochondrial CaMKII (mtCaMKII), whereas mtCaMKII phosphorylated MCU at the regulatory site S92 that promotes Ca2+ entry. mtCaMKII was necessary and sufficient for platelet-derived growth factor-induced mitochondrial Ca2+ uptake. This effect was dependent on MCU. mtCaMKII and MCU inhibition abrogated VSMC migration and mitochondrial translocation to the leading edge. Overexpression of wild-type MCU, but not MCU S92A, mutant in MCU-/- VSMC rescued migration and mitochondrial mobility. Inhibition of microtubule, but not of actin assembly, blocked mitochondrial mobility. The outer mitochondrial membrane GTPase Miro-1 promotes mitochondrial mobility via microtubule transport but arrests it in subcellular domains of high Ca2+ concentrations. In Miro-1-/- VSMC, mitochondrial mobility and VSMC migration were abolished, and overexpression of mtCaMKII or a CaMKII inhibitory peptide in mitochondria (mtCaMKIIN) had no effect. Consistently, inhibition of mtCaMKII increased and prolonged cytosolic Ca2+ transients. mtCaMKII inhibition diminished phosphorylation of focal adhesion kinase and myosin light chain, leading to reduced focal adhesion turnover and cytoskeletal remodeling. In a transgenic model of selective mitochondrial CaMKII inhibition in VSMC, neointimal hyperplasia was significantly reduced after vascular injury.
Conclusions: These findings identify mitochondrial CaMKII as a key regulator of mitochondrial Ca2+ uptake via MCU, thereby controlling mitochondrial translocation and VSMC migration after vascular injury.
Keywords: calcium; calcium-calmodulin-dependent protein kinase type 2; hyperplasia; microtubules; mitochondria; neointima.
© 2018 American Heart Association, Inc.
Conflict of interest statement
Figures



Comment in
-
Smooth Muscle Cells Move With Mitochondria.Arterioscler Thromb Vasc Biol. 2018 Jun;38(6):1255-1257. doi: 10.1161/ATVBAHA.118.311085. Arterioscler Thromb Vasc Biol. 2018. PMID: 29793991 Free PMC article. No abstract available.
References
-
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE Investigators S. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. The New England journal of medicine. 2003;349:1315–1323. - PubMed
-
- Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

