Experimental Right Ventricular Hypertension Induces Regional β1-Integrin-Mediated Transduction of Hypertrophic and Profibrotic Right and Left Ventricular Signaling
- PMID: 29599211
- PMCID: PMC5907585
- DOI: 10.1161/JAHA.117.007928
Experimental Right Ventricular Hypertension Induces Regional β1-Integrin-Mediated Transduction of Hypertrophic and Profibrotic Right and Left Ventricular Signaling
Abstract
Background: Development of right ventricular (RV) hypertension eventually contributes to RV and left ventricular (LV) myocardial fibrosis and dysfunction. The molecular mechanisms are not fully elucidated.
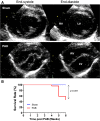
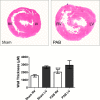
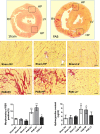
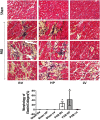
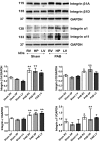
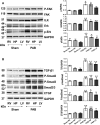
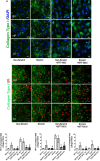
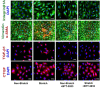
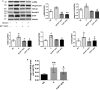
Methods and results: Pulmonary artery banding was used to induce RV hypertension in rats in vivo. Then, we evaluated cardiac function and regional remodeling 6 weeks after pulmonary artery banding. To further elucidate mechanisms responsible for regional cardiac remodeling, we also mimicked RV hypertensive stress by cyclic mechanical stretching applied to confluent cultures of cardiac fibroblasts, isolated from the RV free wall, septal hinge points, and LV free wall. Echocardiography and catheter evaluation demonstrated that rats in the pulmonary artery banding group developed RV hypertension with leftward septal displacement, LV compression, and increased LV end-diastolic pressures. Picrosirius red staining indicated that pulmonary artery banding induced marked RV fibrosis and dysfunction, with prominent fibrosis and elastin deposition at the septal hinge points but less LV fibrosis. These changes were associated with proportionally increased expressions of integrin-β1 and profibrotic signaling proteins, including phosphorylated Smad2/3 and transforming growth factor-β1. Moreover, mechanically stretched fibroblasts also expressed significantly increased levels of α-smooth muscle actin, integrin-β1, transforming growth factor-β1, collagen I deposition, and wrinkle formation on gel assays, consistent with myofibroblast transformation. These changes were not observed in parallel cultures of mechanically stretched fibroblasts, preincubated with the integrin inhibitor (BTT-3033).
Conclusions: Experimentally induced RV hypertension triggers regional RV, hinge-point, and LV integrin β1-dependent mechanotransduction signaling pathways that eventually trigger myocardial fibrosis via transforming growth factor-β1 signaling. Reduced LV fibrosis and preserved global function, despite geometrical and pressure aberrations, suggest a possible elastin-mediated protective mechanism at the septal hinge points.
Keywords: fibrosis; integrin; pressure overload; regional stress.
© 2018 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

References
-
- Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. - PubMed
-
- Belenkie I, Horne SG, Dani R, Smith ER, Tyberg JV. Effects of aortic constriction during experimental acute right ventricular pressure loading: further insights into diastolic and systolic ventricular interaction. Circulation. 1995;92:546–554. - PubMed
-
- Mitchell JR, Whitelaw WA, Sas R, Smith ER, Tyberg JV, Belenkie I. RV filling modulates LV function by direct ventricular interaction during mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289:H549–H557. - PubMed
-
- Damiano RJ Jr, La Follette P Jr, Cox JL, Lowe JE, Santamore WP. Significant left ventricular contribution to right ventricular systolic function. Am J Physiol. 1991;261:H1514–H1524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

