Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule
- PMID: 29599213
- PMCID: PMC6120582
- DOI: 10.1126/science.aaq0939
Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule
Abstract
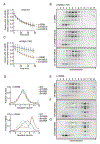
Regulation by the integrated stress response (ISR) converges on the phosphorylation of translation initiation factor eIF2 in response to a variety of stresses. Phosphorylation converts eIF2 from a substrate to a competitive inhibitor of its dedicated guanine nucleotide exchange factor, eIF2B, thereby inhibiting translation. ISRIB, a drug-like eIF2B activator, reverses the effects of eIF2 phosphorylation, and in rodents it enhances cognition and corrects cognitive deficits after brain injury. To determine its mechanism of action, we solved an atomic-resolution structure of ISRIB bound in a deep cleft within decameric human eIF2B by cryo-electron microscopy. Formation of fully active, decameric eIF2B holoenzyme depended on the assembly of two identical tetrameric subcomplexes, and ISRIB promoted this step by cross-bridging a central symmetry interface. Thus, regulation of eIF2B assembly emerges as a rheostat for eIF2B activity that tunes translation during the ISR and that can be further modulated by ISRIB.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


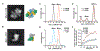


References
-
- Munn DH et al., GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 22, 633–642 (2005). - PubMed
-
- Wortham NC, Martinez M, Gordiyenko Y, Robinson CV, Proud CG, Analysis of the subunit organization of the eIF2B complex reveals new insights into its structure and regulation. The FASEB Journal. 28, 2225–2237 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

