Homologous recombination and the repair of DNA double-strand breaks
- PMID: 29599286
- PMCID: PMC6036207
- DOI: 10.1074/jbc.TM118.000372
Homologous recombination and the repair of DNA double-strand breaks
Abstract
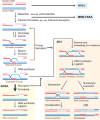
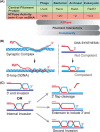
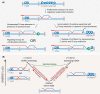
Homologous recombination enables the cell to access and copy intact DNA sequence information in trans, particularly to repair DNA damage affecting both strands of the double helix. Here, we discuss the DNA transactions and enzymatic activities required for this elegantly orchestrated process in the context of the repair of DNA double-strand breaks in somatic cells. This includes homology search, DNA strand invasion, repair DNA synthesis, and restoration of intact chromosomes. Aspects of DNA topology affecting individual steps are highlighted. Overall, recombination is a dynamic pathway with multiple metastable and reversible intermediates designed to achieve DNA repair with high fidelity.
Keywords: DNA damage; DNA endonuclease; DNA helicase; DNA polymerase; DNA recombination; DNA repair; DNA topoisomerase; DNA topology; genomic instability.
© 2018 Wright et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- Kowalczykowski S. C., Hunter N., and Heyer W.-D. (eds) (2016) DNA Recombination, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

