Streptococcus pneumoniae: transmission, colonization and invasion
- PMID: 29599457
- PMCID: PMC5949087
- DOI: 10.1038/s41579-018-0001-8
Streptococcus pneumoniae: transmission, colonization and invasion
Abstract
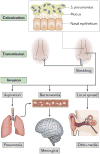
Streptococcus pneumoniae has a complex relationship with its obligate human host. On the one hand, the pneumococci are highly adapted commensals, and their main reservoir on the mucosal surface of the upper airways of carriers enables transmission. On the other hand, they can cause severe disease when bacterial and host factors allow them to invade essentially sterile sites, such as the middle ear spaces, lungs, bloodstream and meninges. Transmission, colonization and invasion depend on the remarkable ability of S. pneumoniae to evade or take advantage of the host inflammatory and immune responses. The different stages of pneumococcal carriage and disease have been investigated in detail in animal models and, more recently, in experimental human infection. Furthermore, widespread vaccination and the resulting immune pressure have shed light on pneumococcal population dynamics and pathogenesis. Here, we review the mechanistic insights provided by these studies on the multiple and varied interactions of the pneumococcus and its host.
Figures



References
-
- Yahiaoui RY, et al. Prevalence and antibiotic resistance of commensal Streptococcus pneumoniae in nine European countries. Future Microbiol. 2016;11:737–744. - PubMed
-
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. - PubMed
-
- Whitney CG, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. - PubMed
-
- Musher D. How contagious are common respiratory tract infections? N Engl J Med. 2003;348:1256–1266. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

