An advanced human in vitro co-culture model for translocation studies across the placental barrier
- PMID: 29599470
- PMCID: PMC5876397
- DOI: 10.1038/s41598-018-23410-6
An advanced human in vitro co-culture model for translocation studies across the placental barrier
Abstract
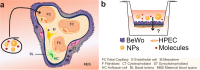
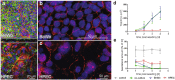
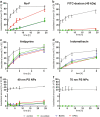
Although various drugs, environmental pollutants and nanoparticles (NP) can cross the human placental barrier and may harm the developing fetus, knowledge on predictive placental transfer rates and the underlying transport pathways is mostly lacking. Current available in vitro placental transfer models are often inappropriate for translocation studies of macromolecules or NPs and do not consider barrier function of placental endothelial cells (EC). Therefore, we developed a human placental in vitro co-culture transfer model with tight layers of trophoblasts (BeWo b30) and placental microvascular ECs (HPEC-A2) on a low-absorbing, 3 µm porous membrane. Translocation studies with four model substances and two polystyrene (PS) NPs across the individual and co-culture layers revealed that for most of these compounds, the trophoblast and the EC layer both demonstrate similar, but not additive, retention capacity. Only the paracellular marker Na-F was substantially more retained by the BeWo layer. Furthermore, simple shaking, which is often applied to mimic placental perfusion, did not alter translocation kinetics compared to static exposure. In conclusion, we developed a novel placental co-culture model, which provides predictive values for translocation of a broad variety of molecules and NPs and enables valuable mechanistic investigations on cell type-specific placental barrier function.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Establishment of an in vitro placental barrier model cultured under physiologically relevant oxygen levels.Mol Hum Reprod. 2020 May 15;26(5):353-365. doi: 10.1093/molehr/gaaa018. Mol Hum Reprod. 2020. PMID: 32159799 Free PMC article.
-
Translocation of positively and negatively charged polystyrene nanoparticles in an in vitro placental model.Toxicol In Vitro. 2015 Oct;29(7):1701-10. doi: 10.1016/j.tiv.2015.07.003. Epub 2015 Jul 3. Toxicol In Vitro. 2015. PMID: 26145586
-
A Novel Human Placental Barrier Model Based on Trophoblast Stem Cells Derived from Human Induced Pluripotent Stem Cells.Tissue Eng Part A. 2020 Jul;26(13-14):780-791. doi: 10.1089/ten.TEA.2019.0342. Epub 2020 May 26. Tissue Eng Part A. 2020. PMID: 32323636
-
Nanoparticle transport across the placental barrier: pushing the field forward!Nanomedicine (Lond). 2016 Apr;11(8):941-57. doi: 10.2217/nnm-2015-0012. Epub 2016 Mar 16. Nanomedicine (Lond). 2016. PMID: 26979802 Review.
-
Research on nanoparticles in human perfused placenta: State of the art and perspectives.Placenta. 2021 Jan 15;104:199-207. doi: 10.1016/j.placenta.2020.12.014. Epub 2020 Dec 28. Placenta. 2021. PMID: 33418345 Review.
Cited by
-
Post-Vaccination Yellow Fever Antiserum Reduces Zika Virus in Embryoid Bodies When Placental Cells are Present.Vaccines (Basel). 2020 Dec 11;8(4):752. doi: 10.3390/vaccines8040752. Vaccines (Basel). 2020. PMID: 33322247 Free PMC article.
-
Environmental and Health Impacts of Graphene and Other Two-Dimensional Materials: A Graphene Flagship Perspective.ACS Nano. 2024 Feb 27;18(8):6038-6094. doi: 10.1021/acsnano.3c09699. Epub 2024 Feb 13. ACS Nano. 2024. PMID: 38350010 Free PMC article. Review.
-
A straightforward cell culture insert model to incorporate biochemical and biophysical stromal properties into transplacental transport studies.Placenta. 2025 Jun 13;166:54-61. doi: 10.1016/j.placenta.2024.09.001. Epub 2024 Sep 5. Placenta. 2025. PMID: 39266436
-
Micro/Nanoplastic Exposure on Placental Health and Adverse Pregnancy Risks: Novel Assessment System Based upon Targeted Risk Assessment Environmental Chemicals Strategy.Toxics. 2024 Jul 30;12(8):553. doi: 10.3390/toxics12080553. Toxics. 2024. PMID: 39195655 Free PMC article.
-
Placental Models for Evaluation of Nanocarriers as Drug Delivery Systems for Pregnancy Associated Disorders.Biomedicines. 2022 Apr 19;10(5):936. doi: 10.3390/biomedicines10050936. Biomedicines. 2022. PMID: 35625672 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

