Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress
- PMID: 29599484
- PMCID: PMC6046049
- DOI: 10.1038/s41386-018-0043-7
Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress
Abstract
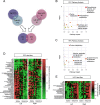
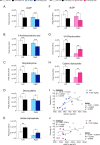
Recently, we have shown that ketamine given prior to stress exposure protects against the development of depressive-like behavior in mice. These data suggest that it may be possible to prevent the induction of affective disorders before they develop by administering prophylactic pharmaceuticals, a relatively nascent and unexplored strategy for psychiatry. Here, we performed metabolomics analysis of brain and plasma following prophylactic ketamine treatment in order to identify markers of stress resilience enhancement. We administered prophylactic ketamine in mice to buffer against fear expression. Following behavioral analyses, untargeted metabolomic profiling was performed on both hemispheres of the prefrontal cortex (PFC) and the hippocampus (HPC), and plasma. We found that prophylactic ketamine attenuated learned fear. Eight metabolites were changed in the PFC and HPC upon ketamine treatment. Purine and pyrimidine metabolism were most significantly changed in the HPC, PFC, and, interestingly, plasma of mice two weeks after prophylactic administration. Moreover, most precursors to inhibitory neurotransmitters were increased whereas precursors to excitatory neurotransmitters were decreased. Strikingly, these long-term metabolomic changes were not observed when no stressor was administered. Our results suggest that prophylactic treatment differentially affects purine and pyrimidine metabolism and neurotransmission in brain and plasma following stress, which may underlie the long-lasting resilience to stress induced by a single injection of ketamine. These data may provide novel targets for prophylactic development, and indicate an interaction effect of prophylactic ketamine and stress. To our knowledge, this is the first study that identifies metabolomic alterations and biomarker candidates for prophylactic ketamine efficacy in mice.
Conflict of interest statement
AM and CTL reported no biomedical financial interests or potential conflicts of interests. CH, AK, NRN, and MAK are employees of BERG LLC and own stock. NRN is a founder of BERG. JCM, RAB, and CAD are named on non-provisional patent applications for the prophylactic use of ketamine against stress-related psychiatric disorders.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

