Daclatasvir plus asunaprevir in treatment-naïve patients with hepatitis C virus genotype 1b infection
- PMID: 29599611
- PMCID: PMC5871831
- DOI: 10.3748/wjg.v24.i12.1361
Daclatasvir plus asunaprevir in treatment-naïve patients with hepatitis C virus genotype 1b infection
Abstract
Aim: To assess daclatasvir plus asunaprevir (DUAL) in treatment-naïve patients from mainland China, Russia and South Korea with hepatitis C virus (HCV) genotype 1b infection.
Methods: Patients were randomly assigned (3:1) to receive 24 wk of treatment with DUAL (daclatasvir 60 mg once daily and asunaprevir 100 mg twice daily) beginning on day 1 of the treatment period (immediate treatment arm) or following 12 wk of matching placebo (placebo-deferred treatment arm). The primary endpoint was a comparison of sustained virologic response at posttreatment week 12 (SVR12) compared with the historical SVR rate for peg-interferon plus ribavirin (70%) among patients in the immediate treatment arm. The first 12 wk of the study were blinded. Safety was assessed in DUAL-treated patients compared with placebo patients during the first 12 wk (double-blind phase), and during 24 wk of DUAL in both arms combined.
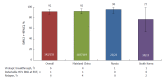
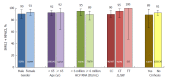
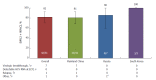
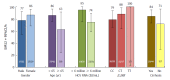
Results: In total, 207 patients were randomly assigned to immediate (n = 155) or placebo-deferred (n = 52) treatment. Most patients were Asian (86%), female (59%) and aged < 65 years (90%). Among them, 13% had cirrhosis, 32% had IL28B non-CC genotypes and 53% had baseline HCV RNA levels of ≥ 6 million IU/mL. Among patients in the immediate treatment arm, SVR12 was achieved by 92% (95% confidence interval: 87.2-96.0), which was significantly higher than the historical comparator rate (70%). SVR12 was largely unaffected by cirrhosis (89%), age ≥ 65 years (92%), male sex (90%), baseline HCV RNA ≥ 6 million (89%) or IL28B non-CC genotypes (96%), although SVR12 was higher among patients without (96%) than among those with (53%) baseline NS5A resistance-associated polymorphisms (at L31 or Y93H). During the double-blind phase, aminotransferase elevations were more common among placebo recipients than among patients receiving DUAL. During 24 wk of DUAL therapy (combined arms), the most common adverse events (≥ 10%) were elevated alanine aminotransferase and upper respiratory tract infection; emergent grade 3-4 laboratory abnormalities were infrequently observed, and all grade 3-4 aminotransferase abnormalities (alanine aminotransferase, n = 9; aspartate transaminase, n = 6) reversed within 8-11 d. Two patients discontinued DUAL treatment; one due to aminotransferase elevations, nausea, and jaundice and the other due to a fatal adverse event unrelated to treatment. There were no treatment-related deaths.
Conclusion: DUAL was well-tolerated during this phase 3 study, and SVR12 with DUAL treatment (92%) exceeded the historical SVR rate for peg-interferon plus ribavirin of 70%.
Keywords: Asunaprevir; Chronic hepatitis C; Daclatasvir; Direct-acting antiviral; Genotype 1b; Liver disease; NS3; NS5A.
Conflict of interest statement
Conflict-of-interest statement: All authors have no conflicts of interest for this manuscript.
Figures
Similar articles
-
A phase 3, open-label study of daclatasvir plus asunaprevir in Asian patients with chronic hepatitis C virus genotype 1b infection who are ineligible for or intolerant to interferon alfa therapies with or without ribavirin.J Gastroenterol Hepatol. 2016 Nov;31(11):1860-1867. doi: 10.1111/jgh.13379. J Gastroenterol Hepatol. 2016. PMID: 27003037 Clinical Trial.
-
All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study.Lancet. 2014 Nov 1;384(9954):1597-605. doi: 10.1016/S0140-6736(14)61059-X. Epub 2014 Jul 28. Lancet. 2014. PMID: 25078304 Clinical Trial.
-
Daclatasvir/asunaprevir/beclabuvir fixed-dose combination in Japanese patients with HCV genotype 1 infection.J Gastroenterol. 2017 Mar;52(3):385-395. doi: 10.1007/s00535-016-1245-6. Epub 2016 Aug 9. J Gastroenterol. 2017. PMID: 27502287 Clinical Trial.
-
Efficacy and safety of a fixed dose combination tablet of asunaprevir + beclabuvir + daclatasvir for the treatment of Hepatitis C.Expert Opin Pharmacother. 2020 Feb;21(3):261-273. doi: 10.1080/14656566.2019.1697674. Epub 2020 Jan 8. Expert Opin Pharmacother. 2020. PMID: 31914336 Review.
-
Effectiveness and safety of daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: Systematic review and meta-analysis.J Gastroenterol Hepatol. 2017 Jan;32(1):45-52. doi: 10.1111/jgh.13587. J Gastroenterol Hepatol. 2017. PMID: 27597318
Cited by
-
Efficacy and Safety of All-oral Emitasvir and Sofosbuvir in Patients with Genotype 1b HCV Infections without Cirrhosis.J Clin Transl Hepatol. 2020 Sep 28;8(3):255-261. doi: 10.14218/JCTH.2020.00031. Epub 2020 Sep 11. J Clin Transl Hepatol. 2020. PMID: 33083247 Free PMC article.
-
Hepatitis C virus cure with direct acting antivirals: Clinical, economic, societal and patient value for China.World J Hepatol. 2019 May 27;11(5):421-441. doi: 10.4254/wjh.v11.i5.421. World J Hepatol. 2019. PMID: 31183003 Free PMC article. Review.
-
The Role of CYPs and Transporters in the Biotransformation and Transport of the Anti-hepatitis C Antiviral Agents Asunaprevir, Daclatasvir, and Beclabuvir: Impact of Liver Disease, Race and Drug-drug Interactions on Safety and Efficacy.Curr Drug Metab. 2024;25(2):96-109. doi: 10.2174/0113892002288832240213095622. Curr Drug Metab. 2024. PMID: 38441017 Review.
-
Hepatitis C Virus: Insights Into Its History, Treatment, Challenges, and Future Directions.Cureus. 2023 Aug 22;15(8):e43924. doi: 10.7759/cureus.43924. eCollection 2023 Aug. Cureus. 2023. PMID: 37614826 Free PMC article. Review.
References
-
- Bennett H, Waser N, Johnston K, Kao JH, Lim YS, Duan ZP, Lee YJ, Wei L, Chen CJ, Sievert W, et al. A review of the burden of hepatitis C virus infection in China, Japan, South Korea and Taiwan. Hepatol Int. 2015;9:378–390. - PubMed
-
- Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31 Suppl 2:61–80. - PubMed
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. - PubMed
-
- Chinese Society of Hepatology, Chinese Medical Association, Wei L; Chinese Society of Infectious Diseases, Chinese Medical Association, Hou JL. [The guideline of prevention and treatment for hepatitis C: a 2015 update] Zhonghua Gan Zang Bing Za Zhi. 2015;23:906–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

