Lipoprotein Lipase Is a Feature of Alternatively-Activated Microglia and May Facilitate Lipid Uptake in the CNS During Demyelination
- PMID: 29599706
- PMCID: PMC5862862
- DOI: 10.3389/fnmol.2018.00057
Lipoprotein Lipase Is a Feature of Alternatively-Activated Microglia and May Facilitate Lipid Uptake in the CNS During Demyelination
Abstract
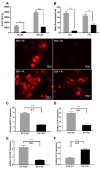
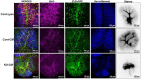
Severe demyelinating disorders of the central nervous system (CNS) such as multiple sclerosis (MS), can be devastating for many young lives. To date, the factors resulting in poor remyelination and repair are not well understood, and reparative therapies that benefit MS patients have yet to be developed. We have previously shown that the activity and abundance of Lipoprotein Lipase (LPL)-the rate-limiting enzyme in the hydrolysis of triglyceride-rich lipoproteins-is increased in Schwann cells and macrophages following nerve crush injury in the peripheral nervous system (PNS), suggesting that LPL may help scavenge myelin-derived lipids. We hypothesized that LPL may play a similar role in the CNS. To test this, mice were immunized with MOG35-55 peptide to induce experimental allergic encephalomyelitis (EAE). LPL activity was increased (p < 0.05) in the brain at 30 days post-injection, coinciding with partial remission of clinical symptoms. Furthermore, LPL abundance and activity was up-regulated (p < 0.05) at the transition between de- and re-myelination in lysolecithin-treated ex vivo cerebellar slices. Since microglia are the key immune effector cells of the CNS we determined the role of LPL in microglia. Lipid uptake was decreased (p < 0.001) in LPL-deficient BV-2 microglial cells compared to WT. In addition, LPL-deficient cells showed dramatically reduced expression of anti-inflammatory markers, YM1 (-22 fold, p < 0.001), and arginase 1 (Arg1; -265 fold, p < 0.001) and increased expression of pro-inflammatory markers, such as iNOS compared to WT cells (+53 fold, p < 0.001). This suggests that LPL is a feature of reparative microglia, further supported by the metabolic and inflammatory profile of LPL-deficient microglia. Taken together, our data strongly suggest that LPL expression is a novel feature of a microglial phenotype that supports remyelination and repair through the clearance of lipid debris. This mechanism may be exploited to develop future reparative therapies for MS and primary neurodegenerative disorders (Alzheimer's disease (AD) and Parkinson's disease).
Keywords: lipid metabolism; lipoprotein lipase (LPL); microglia; multiple sclerosis; myelination.
Figures





Similar articles
-
Systemic TLR2 tolerance enhances central nervous system remyelination.J Neuroinflammation. 2019 Jul 27;16(1):158. doi: 10.1186/s12974-019-1540-2. J Neuroinflammation. 2019. PMID: 31351476 Free PMC article.
-
Genetic Variants of Lipoprotein Lipase and Regulatory Factors Associated with Alzheimer's Disease Risk.Int J Mol Sci. 2020 Nov 6;21(21):8338. doi: 10.3390/ijms21218338. Int J Mol Sci. 2020. PMID: 33172164 Free PMC article. Review.
-
Neuronal injury in chronic CNS inflammation.Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
-
Reduced Angiopoietin-Like 4 Expression in Multiple Sclerosis Lesions Facilitates Lipid Uptake by Phagocytes via Modulation of Lipoprotein-Lipase Activity.Front Immunol. 2019 May 3;10:950. doi: 10.3389/fimmu.2019.00950. eCollection 2019. Front Immunol. 2019. PMID: 31130950 Free PMC article.
-
Nogo receptor expression in microglia/macrophages during experimental autoimmune encephalomyelitis progression.Neural Regen Res. 2018 May;13(5):896-907. doi: 10.4103/1673-5374.232488. Neural Regen Res. 2018. PMID: 29863021 Free PMC article.
Cited by
-
Cells of the adult human heart.Nature. 2020 Dec;588(7838):466-472. doi: 10.1038/s41586-020-2797-4. Epub 2020 Sep 24. Nature. 2020. PMID: 32971526 Free PMC article.
-
Fundamental Neurochemistry Review: Lipids across microglial states.J Neurochem. 2025 Jan;169(1):e16259. doi: 10.1111/jnc.16259. J Neurochem. 2025. PMID: 39696753 Free PMC article. Review.
-
Lipid droplet accumulation in microglia and their potential roles.Lipids Health Dis. 2025 Jun 14;24(1):215. doi: 10.1186/s12944-025-02633-3. Lipids Health Dis. 2025. PMID: 40514678 Free PMC article. Review.
-
Lipid and Lipoprotein Metabolism in Microglia.Front Physiol. 2020 Apr 28;11:393. doi: 10.3389/fphys.2020.00393. eCollection 2020. Front Physiol. 2020. PMID: 32411016 Free PMC article. Review.
-
TREM2 inhibition triggers antitumor cell activity of myeloid cells in glioblastoma.Sci Adv. 2023 May 12;9(19):eade3559. doi: 10.1126/sciadv.ade3559. Epub 2023 May 12. Sci Adv. 2023. PMID: 37172094 Free PMC article.
References
-
- Amor S., Groome N., Linington C., Morris M. M., Dornmair K., Gardinier M. V., et al. (1994). Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J. Immunol. 153, 4349–4356. - PubMed
-
- Bessesen D. H., Richards C. L., Etienne J., Goers J. W., Eckel R. H. (1993). Spinal cord of the rat contains more lipoprotein lipase than other brain regions. J. Lipid Res. 34, 229–238. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

