Administration of the Antioxidant N-Acetyl-Cysteine in Pregnant Mice Has Long-Term Positive Effects on Metabolic and Behavioral Endpoints of Male and Female Offspring Prenatally Exposed to a High-Fat Diet
- PMID: 29599711
- PMCID: PMC5862866
- DOI: 10.3389/fnbeh.2018.00048
Administration of the Antioxidant N-Acetyl-Cysteine in Pregnant Mice Has Long-Term Positive Effects on Metabolic and Behavioral Endpoints of Male and Female Offspring Prenatally Exposed to a High-Fat Diet
Abstract
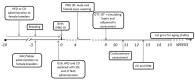
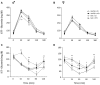
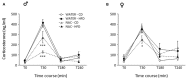
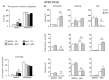
A growing body of evidence suggests the consumption of high-fat diet (HFD) during pregnancy to model maternal obesity and the associated increase in oxidative stress (OS), might act as powerful prenatal stressors, leading to adult stress-related metabolic or behavioral disorders. We hypothesized that administration of antioxidants throughout gestation might counteract the negative effects of prenatal exposure to metabolic challenges (maternal HFD feeding during pregnancy) on the developing fetus. In this study, female C57BL/6J mice were fed HFD for 13 weeks (from 5-weeks of age until delivery) and were exposed to the N-acetyl-cysteine (NAC) antioxidant from 10-weeks of age until right before delivery. Body weight of the offspring was assessed following birth, up to weaning and at adulthood. The metabolic, neuroendocrine and emotional profile of the adult offspring was tested at 3-months of age. Prenatal HFD increased mother's body weight and offspring's weight at the time of weaning, when administered in conjunction with NAC. In females, NAC administration reduced high levels of leptin resulting from prenatal HFD. Prenatal NAC administration also resulted in greater glucose tolerance and insulin sensitivity while increasing adiponectin levels, as well as increasing exploratory behavior, an effect accompanied by reduced plasma corticosterone levels in response to restraint stress. Analysis of glutathione levels in the hypothalamus and in brown adipose tissue indicates that, while HFD administration to pregnant dams led to reduced levels of glutathione in the offspring, as in the male hypothalamus, NAC was able to revert this effect and to increase glutathione levels both in the periphery (Brown Adipose Tissue, both males and females) and in the central nervous system (males). Overall, results from this study indicate that the body redox milieu should be tightly regulated during fetal life and that buffering OS during pregnancy can have important long-term consequences on metabolic and behavioral endpoints.
Keywords: N-acetyl-cysteine; behavior; glutathione; high-fat diet; metabolism; mice; oxidative stress; pregnancy.
Figures




References
-
- Balansky R., Izzotti A., Scatolini L., D’Agostini F., De Flora S. (1996). Induction by carcinogens and chemoprevention by N-acetylcysteine of adducts to mitochondrial DNA in rat organs. Cancer Res. 56, 1642–1647. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

