Metabotropic Glutamate2 Receptors Play a Key Role in Modulating Head Twitches Induced by a Serotonergic Hallucinogen in Mice
- PMID: 29599719
- PMCID: PMC5862811
- DOI: 10.3389/fphar.2018.00208
Metabotropic Glutamate2 Receptors Play a Key Role in Modulating Head Twitches Induced by a Serotonergic Hallucinogen in Mice
Abstract
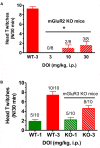
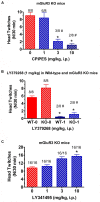
There is substantial evidence that glutamate can modulate the effects of 5-hydroxytryptamine2A (5-HT2A) receptor activation through stimulation of metabotropic glutamate2/3 (mGlu2/3) receptors in the prefrontal cortex. Here we show that constitutive deletion of the mGlu2 gene profoundly attenuates an effect of 5-HT2A receptor activation using the mouse head twitch response (HTR). MGlu2 and mGlu3 receptor knockout (KO) as well as age-matched ICR (CD-1) wild type (WT) mice were administered (±)1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and observed for head twitch activity. DOI failed to produce significant head twitches in mGlu2 receptor KO mice at a dose 10-fold higher than the peak effective dose in WT or mGlu3 receptor KO mice. In addition, the mGlu2/3 receptor agonist LY379268, and the mGlu2 receptor positive allosteric modulator (PAM) CBiPES, potently blocked the HTR to DOI in WT and mGlu3 receptor KO mice. Conversely, the mGlu2/3 receptor antagonist LY341495 (10 mg/kg) increased the HTR produced by DOI (3 mg/kg) in mGlu3 receptor KO mice. Finally, the mGlu2 receptor potentiator CBiPES was able to attenuate the increase in the HTR produced by LY341495 in mGlu3 receptor KO mice. Taken together, all of these results are consistent with the hypothesis that that DOI-induced head twitches are modulated by mGlu2 receptor activation. These results also are in keeping with a critical autoreceptor function for mGlu2 receptors in the prefrontal cortex with differential effects of acute vs. chronic perturbation (e.g., constitutive mGlu2 receptor KO mice). The robust attenuation of DOI-induced head twitches in the mGlu2 receptor KO mice appears to reflect the critical role of glutamate in ongoing regulation of 5-HT2A receptors in the prefrontal cortex. Future experiments with inducible knockouts for the mGlu2 receptor and/or selective mGlu3 receptor agonists/PAMs/antagonists could provide an important tools in understanding glutamatergic modulation of prefrontal cortical 5-HT2A receptor function.
Keywords: 5-hydroxytryptamine2A (5-HT2A) receptors; CBiPES; DOI; LY341495; LY379268; head twitches; prefrontal cortex (PFC); transgenic mice.
Figures



Similar articles
-
Chronic treatment with a metabotropic mGlu2/3 receptor agonist diminishes behavioral response to a phenethylamine hallucinogen.Psychopharmacology (Berl). 2019 Feb;236(2):821-830. doi: 10.1007/s00213-018-5118-y. Epub 2018 Nov 17. Psychopharmacology (Berl). 2019. PMID: 30448990 Free PMC article.
-
Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice.Mol Pharmacol. 2009 Aug;76(2):379-87. doi: 10.1124/mol.109.056580. Epub 2009 May 13. Mol Pharmacol. 2009. PMID: 19439499
-
β2-Adrenergic Receptor Activation Suppresses the Rat Phenethylamine Hallucinogen-Induced Head Twitch Response: Hallucinogen-Induced Excitatory Post-synaptic Potentials as a Potential Substrate.Front Pharmacol. 2018 Feb 8;9:89. doi: 10.3389/fphar.2018.00089. eCollection 2018. Front Pharmacol. 2018. PMID: 29472863 Free PMC article.
-
Interactions of Hallucinogens with the Glutamatergic System: Permissive Network Effects Mediated Through Cortical Layer V Pyramidal Neurons.Curr Top Behav Neurosci. 2018;36:107-135. doi: 10.1007/7854_2017_480. Curr Top Behav Neurosci. 2018. PMID: 28831734 Review.
-
Advances in translating mGlu2 and mGlu3 receptor selective allosteric modulators as breakthrough treatments for affective disorders and alcohol use disorder.Pharmacol Biochem Behav. 2022 Sep;219:173450. doi: 10.1016/j.pbb.2022.173450. Epub 2022 Aug 18. Pharmacol Biochem Behav. 2022. PMID: 35988792 Free PMC article. Review.
Cited by
-
Role of mGlu2 in the 5-HT2A receptor-dependent antipsychotic activity of clozapine in mice.Psychopharmacology (Berl). 2018 Nov;235(11):3149-3165. doi: 10.1007/s00213-018-5015-4. Epub 2018 Sep 12. Psychopharmacology (Berl). 2018. PMID: 30209534 Free PMC article.
-
Chronic treatment with a metabotropic mGlu2/3 receptor agonist diminishes behavioral response to a phenethylamine hallucinogen.Psychopharmacology (Berl). 2019 Feb;236(2):821-830. doi: 10.1007/s00213-018-5118-y. Epub 2018 Nov 17. Psychopharmacology (Berl). 2019. PMID: 30448990 Free PMC article.
-
Psilocybin targets a common molecular mechanism for cognitive impairment and increased craving in alcoholism.Sci Adv. 2021 Nov 19;7(47):eabh2399. doi: 10.1126/sciadv.abh2399. Epub 2021 Nov 17. Sci Adv. 2021. PMID: 34788104 Free PMC article.
-
Catalysts for change: the cellular neurobiology of psychedelics.Mol Biol Cell. 2021 Jun 1;32(12):1135-1144. doi: 10.1091/mbc.E20-05-0340. Mol Biol Cell. 2021. PMID: 34043427 Free PMC article. Review.
-
Psilocybin-induced default mode network hypoconnectivity is blunted in alcohol-dependent rats.Transl Psychiatry. 2023 Dec 14;13(1):392. doi: 10.1038/s41398-023-02690-1. Transl Psychiatry. 2023. PMID: 38097569 Free PMC article. Clinical Trial.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

