Evaluation of the Efficacy and Cross-Protective Immunity of Live-Attenuated Chimeric PCV1-2b Vaccine Against PCV2b and PCV2d Subtype Challenge in Pigs
- PMID: 29599761
- PMCID: PMC5862802
- DOI: 10.3389/fmicb.2018.00455
Evaluation of the Efficacy and Cross-Protective Immunity of Live-Attenuated Chimeric PCV1-2b Vaccine Against PCV2b and PCV2d Subtype Challenge in Pigs
Abstract
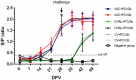
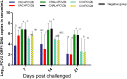
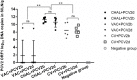
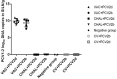
Porcine circovirus type 2 (PCV2) commercial vaccines are either inactivated PCV2 isolates or subunit vaccine based on the Cap protein of PCV2. Currently, no live-attenuated vaccines are yet available. Although the predominant circulating subtype worldwide is PCV2b, the emerging PCV2d subtype is also increasingly associated with PCV disease. In this study, piglets were inoculated with a live-attenuated chimeric PCV1-2b vaccine before challenged with PCV2b and PCV2d isolates. Thirty-two piglets were randomly divided into seven groups: negative (sham-vaccinated, sham-challenged), VAC+PCV2b (PCV1-2b vaccinated, PCV2b-challenged), VAC+PCV2d (PCV1-2b vaccinated, PCV2d-challenged), CHAL+PCV2b (sham-vaccinated, PCV2b-challenged), CHAL+PCV2d (sham-vaccinated, PCV2d-challenged), CV+PCV2b (commercial-vaccinated, PCV2b-challenged), and CV+PCV2d (commercial-vaccinated, PCV2d-challenged). The results showed that vaccinated challenged groups demonstrated high levels of anti-PCV2 antibody and reduced PCV2b and PCV2d loads both in serum and nasal swabs compared with the challenge-only groups. PCV2 DNA was detected in the superficial inguinal lymph nodes of only one pig in each of the VAC+PCV2b and VAC+PCV2d groups (group mean values, 101.81 and 101.77 genomic copies/g, respectively), which was significantly lower than those in CHAL+PCV2b and CHAL+PCV2d animals (group mean values, 1011.65 and 1010.60 genomic copies/g, respectively; P < 0.01). In addition, PCV2 DNA in each of the VAC+PCV2b and VAC+PCV2d groups was significantly lower than those in CV+PCV2b and CV+PCV2d animals (group mean values, 108.47 and 108.34 genomic copies/g, respectively; P < 0.01), indicating that the live-attenuated PCV1-2b vaccine was more effective than commercial vaccine. The live-attenuated PCV1-2b vaccine was effective in reducing PCV2b/PCV2d viremia, shedding, and tissue viral loads in vaccinated challenged pigs compared with challenge-only piglets, indicating that the PCV1-2b prototype vaccine is a good candidate for a live-attenuated vaccine against both PCV2b and PCV2d subtypes. And we first revealed that the live-attenuated PCV1-2b vaccine could protect piglets from challenge with either PCV2b or PCV2d equivalently.
Keywords: cross-protective immunity; live-attenuated chimeric porcine circovirus 1-2b; porcine circovirus 2b; porcine circovirus 2d; vaccine.
Figures



References
-
- Aida J., Izumiyama-Shimomura N., Nakamura K., Ishii A., Ishikawa N., Honma N., et al. (2007). Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum. Pathol. 38 1192–1200. 10.1016/j.humpath.2006.11.023 - DOI - PubMed
-
- Beach N. M., Ramamoorthy S., Opriessnig T., Wu S. Q., Meng X. J. (2010). Novel chimeric porcine circovirus (PCV) with the capsid gene of the emerging PCV2b subtype cloned in the genomic backbone of the non-pathogenic PCV1 is attenuated in vivo and induces protective and cross-protective immunity against PCV2b and PCV2a subtypes in pigs. Vaccine 29 221–232. 10.1016/j.vaccine.2010.10.050 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

