Mother's Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity
- PMID: 29599768
- PMCID: PMC5863526
- DOI: 10.3389/fimmu.2018.00361
Mother's Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity
Abstract
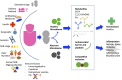
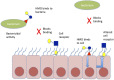
Breast milk is the perfect nutrition for infants, a result of millions of years of evolution. In addition to providing a source of nutrition, breast milk contains a diverse array of microbiota and myriad biologically active components that are thought to guide the infant's developing mucosal immune system. It is believed that bacteria from the mother's intestine may translocate to breast milk and dynamically transfer to the infant. Such interplay between mother and her infant is a key to establishing a healthy infant intestinal microbiome. These intestinal bacteria protect against many respiratory and diarrheal illnesses, but are subject to environmental stresses such as antibiotic use. Orchestrating the development of the microbiota are the human milk oligosaccharides (HMOs), the synthesis of which are partially determined by the maternal genotype. HMOs are thought to play a role in preventing pathogenic bacterial adhesion though multiple mechanisms, while also providing nutrition for the microbiome. Extracellular vesicles (EVs), including exosomes, carry a diverse cargo, including mRNA, miRNA, and cytosolic and membrane-bound proteins, and are readily detectable in human breast milk. Strongly implicated in cell-cell signaling, EVs could therefore may play a further role in the development of the infant microbiome. This review considers the emerging role of breast milk microbiota, bioactive HMOs, and EVs in the establishment of the neonatal microbiome and the consequent potential for modulation of neonatal immune system development.
Keywords: breast milk; breast milk microbiome; exosomes; extracellular vesicles; human milk oligosaccharides; infant microbiome; microbiome; microbiota.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

