Discovering Macrophage Functions Using In Vivo Optical Imaging Techniques
- PMID: 29599778
- PMCID: PMC5863475
- DOI: 10.3389/fimmu.2018.00502
Discovering Macrophage Functions Using In Vivo Optical Imaging Techniques
Abstract
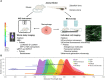
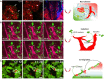
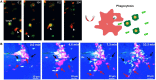
Macrophages are an important component of host defense and inflammation and play a pivotal role in immune regulation, tissue remodeling, and metabolic regulation. Since macrophages are ubiquitous in human bodies and have versatile physiological functions, they are involved in virtually every disease, including cancer, diabetes, multiple sclerosis, and atherosclerosis. Molecular biological and histological methods have provided critical information on macrophage biology. However, many in vivo dynamic behaviors of macrophages are poorly understood and yet to be discovered. A better understanding of macrophage functions and dynamics in pathogenesis will open new opportunities for better diagnosis, prognostic assessment, and therapeutic intervention. In this article, we will review the advances in macrophage tracking and analysis with in vivo optical imaging in the context of different diseases. Moreover, this review will cover the challenges and solutions for optical imaging techniques during macrophage intravital imaging.
Keywords: bioluminescence imaging; cell tracking; function; intravital microscopy; macrophage; optical imaging.
Figures

Similar articles
-
Imaging macrophages with nanoparticles.Nat Mater. 2014 Feb;13(2):125-38. doi: 10.1038/nmat3780. Nat Mater. 2014. PMID: 24452356 Review.
-
Surface-Modified Macrophages Facilitate Tracking of Breast Cancer-Immune Interactions.ACS Chem Biol. 2018 Aug 17;13(8):2339-2346. doi: 10.1021/acschembio.8b00509. Epub 2018 Jun 12. ACS Chem Biol. 2018. PMID: 29856604 Free PMC article.
-
Real-time high-resolution magnetic resonance tracking of macrophage subpopulations in a murine inflammation model: a pilot study with a commercially available cryogenic probe.Contrast Media Mol Imaging. 2013 Mar-Apr;8(2):193-203. doi: 10.1002/cmmi.1516. Contrast Media Mol Imaging. 2013. PMID: 23281292
-
Fluorescent Phosphorus Dendrimer as a Spectral Nanosensor for Macrophage Polarization and Fate Tracking in Spinal Cord Injury.Macromol Biosci. 2015 Nov;15(11):1523-34. doi: 10.1002/mabi.201500150. Epub 2015 Jul 14. Macromol Biosci. 2015. PMID: 26175127
-
Cousins at work: How combining medical with optical imaging enhances in vivo cell tracking.Int J Biochem Cell Biol. 2018 Sep;102:40-50. doi: 10.1016/j.biocel.2018.06.008. Epub 2018 Jun 28. Int J Biochem Cell Biol. 2018. PMID: 29960079 Free PMC article. Review.
Cited by
-
Autofluorescence Imaging of 3D Tumor-Macrophage Microscale Cultures Resolves Spatial and Temporal Dynamics of Macrophage Metabolism.Cancer Res. 2020 Dec 1;80(23):5408-5423. doi: 10.1158/0008-5472.CAN-20-0831. Epub 2020 Oct 22. Cancer Res. 2020. PMID: 33093167 Free PMC article.
-
Biomechanical Contributions to Macrophage Activation in the Tumor Microenvironment.Front Oncol. 2020 May 20;10:787. doi: 10.3389/fonc.2020.00787. eCollection 2020. Front Oncol. 2020. PMID: 32509583 Free PMC article. Review.
-
Machine Learning-Assisted Near-Infrared Spectral Fingerprinting for Macrophage Phenotyping.ACS Nano. 2024 Aug 27;18(34):22874-22887. doi: 10.1021/acsnano.4c03387. Epub 2024 Aug 15. ACS Nano. 2024. PMID: 39148286
-
Intravital lipid droplet labeling and imaging reveals the phenotypes and functions of individual macrophages in vivo.J Lipid Res. 2022 May;63(5):100207. doi: 10.1016/j.jlr.2022.100207. Epub 2022 Apr 6. J Lipid Res. 2022. PMID: 35398040 Free PMC article.
-
Macrophages: an indispensable piece of ovarian health.Biol Reprod. 2021 Mar 11;104(3):527-538. doi: 10.1093/biolre/ioaa219. Biol Reprod. 2021. PMID: 33274732 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

