Bi-Allelic Mutations in STXBP2 Reveal a Complementary Role for STXBP1 in Cytotoxic Lymphocyte Killing
- PMID: 29599780
- PMCID: PMC5862791
- DOI: 10.3389/fimmu.2018.00529
Bi-Allelic Mutations in STXBP2 Reveal a Complementary Role for STXBP1 in Cytotoxic Lymphocyte Killing
Abstract
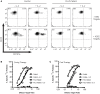
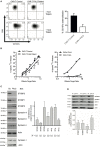
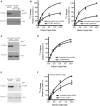
The ability of cytotoxic lymphocytes (CL) to eliminate virus-infected or cancerous target cells through the granule exocytosis death pathway is critical to immune homeostasis. Congenital loss of CL function due to bi-allelic mutations in PRF1, UNC13D, STX11, or STXBP2 leads to a potentially fatal immune dysregulation, familial haemophagocytic lymphohistiocytosis (FHL). This occurs due to the failure of CLs to release functional pore-forming protein perforin and, therefore, inability to kill the target cell. Bi-allelic mutations in partner proteins STXBP2 or STX11 impair CL cytotoxicity due to failed docking/fusion of cytotoxic secretory granules with the plasma membrane. One unique feature of STXBP2- and STX11-deficient patient CLs is that their short-term in vitro treatment with a low concentration of IL-2 partially or completely restores natural killer (NK) cell degranulation and cytotoxicity, suggesting the existence of a secondary, yet unknown, pathway for secretory granule exocytosis. In the current report, we studied NK and T-cell function in an individual with late presentation of FHL due to hypomorphic bi-allelic mutations in STXBP2. Intriguingly, in addition to the expected alterations in the STXBP2 and STX11 proteins, we also observed a concomitant significant reduction in the expression of homologous STXBP1 protein and its partner STX1, which had never been implicated in CL function. Further analysis of human NK and T cells demonstrated a functional role for the STXBP1/STX1 axis in NK and CD8+ T-cell cytotoxicity, where it appears to be responsible for as much as 50% of their cytotoxic activity. This discovery suggests a unique and previously unappreciated interplay between STXBP/Munc proteins regulating the same essential granule exocytosis pathway.
Keywords: Munc18-1; Munc18-2; apoptosis; cytotoxic T cells; cytotoxic lymphocytes; familial haemophagocytic lymphohistiocytosis; immunodeficiency; natural killer cells.
Figures
Similar articles
-
STXBP2-R190C Variant in a Patient With Neonatal Hemophagocytic Lymphohistiocytosis (HLH) and G6PD Deficiency Reveals a Critical Role of STXBP2 Domain 2 on Granule Exocytosis.Front Immunol. 2020 Oct 8;11:545414. doi: 10.3389/fimmu.2020.545414. eCollection 2020. Front Immunol. 2020. PMID: 33162974 Free PMC article.
-
Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells.J Clin Invest. 2009 Dec;119(12):3765-73. doi: 10.1172/JCI40732. Epub 2009 Nov 2. J Clin Invest. 2009. PMID: 19884660 Free PMC article.
-
Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production.Blood. 2013 Feb 21;121(8):1345-56. doi: 10.1182/blood-2012-07-442558. Epub 2013 Jan 2. Blood. 2013. PMID: 23287865
-
A unique SNARE machinery for exocytosis of cytotoxic granules and platelets granules.Mol Membr Biol. 2015;32(4):120-6. doi: 10.3109/09687688.2015.1079934. Mol Membr Biol. 2015. PMID: 26508555 Review.
-
Digenic Inheritance: Evidence and Gaps in Hemophagocytic Lymphohistiocytosis.Front Immunol. 2021 Nov 17;12:777851. doi: 10.3389/fimmu.2021.777851. eCollection 2021. Front Immunol. 2021. PMID: 34868048 Free PMC article. Review.
Cited by
-
Identification of cuproptosis and immune-related gene prognostic signature in lung adenocarcinoma.Front Immunol. 2023 Aug 9;14:1179742. doi: 10.3389/fimmu.2023.1179742. eCollection 2023. Front Immunol. 2023. PMID: 37622116 Free PMC article.
-
Molecular analysis of the novel L243R mutation in STXBP2 reveals impairment of degranulation activity.Int J Hematol. 2020 Mar;111(3):440-450. doi: 10.1007/s12185-019-02796-7. Epub 2019 Dec 21. Int J Hematol. 2020. PMID: 31865540
-
Genotype and phenotype spectrum of 10 children with STXBP1 gene-related encephalopathy and epilepsy.Front Pediatr. 2022 Nov 11;10:1010886. doi: 10.3389/fped.2022.1010886. eCollection 2022. Front Pediatr. 2022. PMID: 36440324 Free PMC article.
-
Role of Genetic Polymorphism Present in Macrophage Activation Syndrome Pathway in Post Mortem Biopsies of Patients with COVID-19.Viruses. 2022 Jul 31;14(8):1699. doi: 10.3390/v14081699. Viruses. 2022. PMID: 36016321 Free PMC article.
-
Different Munc18 proteins mediate baseline and stimulated airway mucin secretion.JCI Insight. 2019 Mar 21;4(6):e124815. doi: 10.1172/jci.insight.124815. eCollection 2019 Mar 21. JCI Insight. 2019. PMID: 30721150 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

