Thioredoxin-1 Protects Bone Marrow-Derived Mesenchymal Stromal Cells from Hyperoxia-Induced Injury In Vitro
- PMID: 29599892
- PMCID: PMC5828533
- DOI: 10.1155/2018/1023025
Thioredoxin-1 Protects Bone Marrow-Derived Mesenchymal Stromal Cells from Hyperoxia-Induced Injury In Vitro
Abstract
Background: The poor survival rate of mesenchymal stromal cells (MSC) transplanted into recipient lungs greatly limits their therapeutic efficacy for diseases like bronchopulmonary dysplasia (BPD). The aim of this study is to evaluate the effect of thioredoxin-1 (Trx-1) overexpression on improving the potential for bone marrow-derived mesenchymal stromal cells (BMSCs) to confer resistance against hyperoxia-induced cell injury.
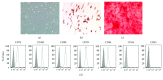
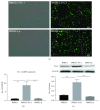
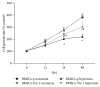
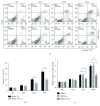
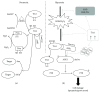
Methods: 80% O2 was used to imitate the microenvironment surrounding-transplanted cells in the hyperoxia-induced lung injury in vitro. BMSC proliferation and apoptotic rates and the levels of reactive oxygen species (ROS) were measured. The effects of Trx-1 overexpression on the level of antioxidants and growth factors were investigated. We also investigated the activation of apoptosis-regulating kinase-1 (ASK1) and p38 mitogen-activated protein kinases (MAPK).
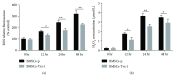
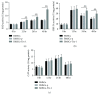
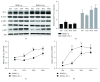
Result: Trx-1 overexpression significantly reduced hyperoxia-induced BMSC apoptosis and increased cell proliferation. We demonstrated that Trx-1 overexpression upregulated the levels of superoxide dismutase and glutathione peroxidase as well as downregulated the production of ROS. Furthermore, we illustrated that Trx-1 protected BMSCs against hyperoxic injury via decreasing the ASK1/P38 MAPK activation rate.
Conclusion: These results demonstrate that Trx-1 overexpression improved the ability of BMSCs to counteract hyperoxia-induced injury, thus increasing their potential to treat hyperoxia-induced lung diseases such as BPD.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

