Reactivation of hepatitis B after liver transplantation: Current knowledge, molecular mechanisms and implications in management
- PMID: 29599899
- PMCID: PMC5871856
- DOI: 10.4254/wjh.v10.i3.352
Reactivation of hepatitis B after liver transplantation: Current knowledge, molecular mechanisms and implications in management
Abstract
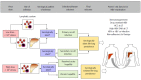
Chronic hepatitis B (CHB) is a major global health problem affecting an estimated 350 million people with more than 786000 individuals dying annually due to complications, such as cirrhosis, liver failure and hepatocellular carcinoma (HCC). Liver transplantation (LT) is considered gold standard for treatment of hepatitis B virus (HBV)-related liver failure and HCC. However, post-transplant viral reactivation can be detrimental to allograft function, leading to poor survival. Prophylaxis with high-dose hepatitis B immunoglobulin (HBIG) and anti-viral drugs have achieved remarkable progress in LT by suppressing viral replication and improving long-term survival. The combination of lamivudine (LAM) plus HBIG has been for many years the most widely used. However, life-long HBIG use is both cumbersome and costly, whereas long-term use of LAM results in resistant virus. Recently, in an effort to develop HBIG-free protocols, high potency nucleos(t)ide analogues, such as Entecavir or Tenofovir, have been tried either as monotherapy or in combination with low-dose HBIG with excellent results. Current focus is on novel antiviral targets, especially for covalently closed circular DNA (cccDNA), in an effort to eradicate HBV infection instead of viral suppression. However, there are several other molecular mechanisms through which HBV may reactivate and need equal attention. The purpose of this review is to address post-LT HBV reactivation, its risk factors, underlying molecular mechanisms, and recent advancements and future of anti-viral therapy.
Keywords: Antivirals; Hepatitis B immunoglobulin; Hepatitis B virus; Liver transplantation; Prophylaxis; Reactivation; Recurrence.
Conflict of interest statement
Conflict-of-interest statement: All authors declare no potential conflicts of interest related to this manuscript. This manuscript is not supported by any grants/funding.
Figures



References
-
- Durantel D, Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J Hepatol. 2016;64:S117–S131. - PubMed
-
- Song GW, Ahn CS, Lee SG, Hwang S, Kim KH, Moon DB, Ha TY, Jung DH, Park GC, Kang SH, et al. Correlation between risk of hepatitis B virus recurrence and tissue expression of covalently closed circular DNA in living donor liver transplant recipients treated with high-dose hepatitis B immunoglobulin. Transplant Proc. 2014;46:3548–3553. - PubMed
-
- Wu D, Ning Q. Toward a Cure for Hepatitis B Virus Infection: Combination Therapy Involving Viral Suppression and Immune Modulation and Long-term Outcome. J Infect Dis. 2017;216:S771–S777. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

