The photoreactive free radical in eumelanin
- PMID: 29600273
- PMCID: PMC5873843
- DOI: 10.1126/sciadv.aaq1293
The photoreactive free radical in eumelanin
Abstract
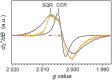
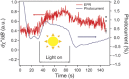
Melanin is the primary photoprotecting pigment in humans as well as being implicated in the development of deadly melanoma. The material also conducts electricity and has thus become a bioelectronic model for proton-to-electron transduction. Central to these phenomena are its spin properties-notably two linked species derived from carbon-centered and semiquinone radicals. Using a novel in situ photoinduced electron paramagnetic resonance technique with simultaneous electrical measurements, we have elucidated for the first time the distinct photoreactivity of the two different radical species. We find that the production of the semiquinone is light- and water-driven, explaining the electrical properties and revealing biologically relevant photoreactivity.
Figures
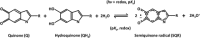

References
-
- G. Prota, Melanins and Melanogenesis (Academic Press, 1992).
-
- Meredith P., Sarna T., The physical and chemical properties of eumelanin. Pigment Cell Res. 19, 572–594 (2006). - PubMed
-
- Sarna T., Pilas B., Land E. J., Truscott T. G., Interaction of radicals from water radiolysis with melanin. Biochim. Biophys. Acta 883, 162–167 (1986). - PubMed
-
- Lin J. Y., Fisher D. E., Melanocyte biology and skin pigmentation. Nature 445, 843–850 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

