Tumor lysate-based vaccines: on the road to immunotherapy for gallbladder cancer
- PMID: 29600445
- PMCID: PMC6244977
- DOI: 10.1007/s00262-018-2157-5
Tumor lysate-based vaccines: on the road to immunotherapy for gallbladder cancer
Abstract
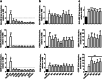
Immunotherapy based on checkpoint blockers has proven survival benefits in patients with melanoma and other malignancies. Nevertheless, a significant proportion of treated patients remains refractory, suggesting that in combination with active immunizations, such as cancer vaccines, they could be helpful to improve response rates. During the last decade, we have used dendritic cell (DC) based vaccines where DCs loaded with an allogeneic heat-conditioned melanoma cell lysate were tested in a series of clinical trials. In these studies, 60% of stage IV melanoma DC-treated patients showed immunological responses correlating with improved survival. Further studies showed that an essential part of the clinical efficacy was associated with the use of conditioned lysates. Gallbladder cancer (GBC) is a high-incidence malignancy in South America. Here, we evaluated the feasibility of producing effective DCs using heat-conditioned cell lysates derived from gallbladder cancer cell lines (GBCCL). By characterizing nine different GBCCLs and several fresh tumor tissues, we found that they expressed some tumor-associated antigens such as CEA, MUC-1, CA19-9, Erb2, Survivin, and several carcinoembryonic antigens. Moreover, heat-shock treatment of GBCCLs induced calreticulin translocation and release of HMGB1 and ATP, both known to act as danger signals. Monocytes stimulated with combinations of conditioned lysates exhibited a potent increase of DC-maturation markers. Furthermore, conditioned lysate-matured DCs were capable of strongly inducing CD4+ and CD8+ T cell activation, in both allogeneic and autologous cell co-cultures. Finally, in vitro stimulated CD8+ T cells recognize HLA-matched GBCCLs. In summary, GBC cell lysate-loaded DCs may be considered for future immunotherapy approaches.
Keywords: CITIM 2017; Dendritic cells; Gallbladder cancer; Immunotherapy; Melanoma; Tumor lysates.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

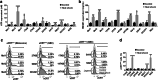


References
-
- Rojas-Sepúlveda D, Gleisner MA, Pereda C, López MN, Salazar-Onfray F. Tumor cell lysates as maturation stimulus and antigen source for therapeutic dendritic cells against gallbladder cancer. Eur J Immunol. 2016;46(S1):1221–1222.
-
- Rojas-Sepúlveda D, Gleisner MA, Pereda C, López MN, Salazar-Onfray F. Tumor lysate loaded dendritic cells induce a T cell specific antitumor response against gallbladder cancer. J Immunol. 2017;198(1 Supplement):79.18.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

