Human Enzymes for Organic Synthesis
- PMID: 29600541
- PMCID: PMC6334177
- DOI: 10.1002/anie.201800678
Human Enzymes for Organic Synthesis
Abstract
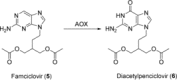
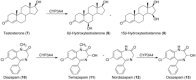
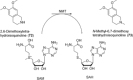
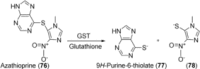
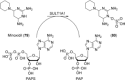
Human enzymes have been widely studied in various disciplines. The number of reactions taking place in the human body is vast, and so is the number of potential catalysts for synthesis. Herein, we focus on the application of human enzymes that catalyze chemical reactions in course of the metabolism of drugs and xenobiotics. Some of these reactions have been explored on the preparative scale. The major field of application of human enzymes is currently drug development, where they are applied for the synthesis of drug metabolites.
Keywords: biocatalysis; biotransformation; drug metabolites; human enzymes; pharmaceutical compounds.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
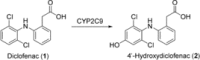

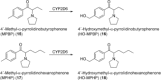
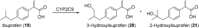
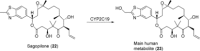
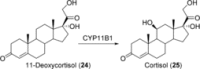
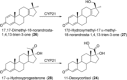
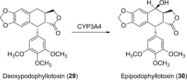
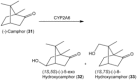
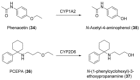



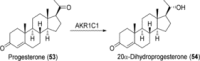
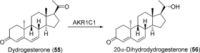

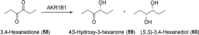
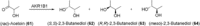
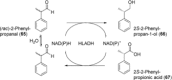
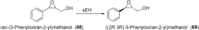
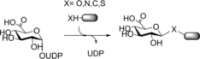
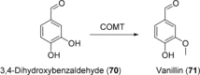
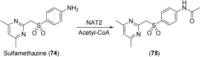
References
-
- Whitesides G. M., Wong C.-H., Angew. Chem. Int. Ed. Engl. 1985, 24, 617–638;
- Angew. Chem. 1985, 97, 617–638.
-
- Schmid A., Dordick J. S., Hauer B., Kiener A., Wubbolts M., Witholt B., Nature 2001, 409, 258–268. - PubMed
-
- Panke S., Wubbolts M., Curr. Opin. Chem. Biol. 2005, 9, 188–194. - PubMed
-
- Bornscheuer U. T., Huisman G. W., Kazlauskas R. J., Lutz S., Moore J. C., Robins K., Nature 2012, 485, 185–194. - PubMed
-
- Nestl B. M., Hammer S. C., Nebel B. A., Hauer B., Angew. Chem. Int. Ed. 2014, 53, 3070–3095; - PubMed
- Angew. Chem. 2014, 126, 3132–3158.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

