The Tablets, Ring, Injections as Options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention
- PMID: 29600595
- PMCID: PMC5876496
- DOI: 10.1002/jia2.25094
The Tablets, Ring, Injections as Options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention
Abstract
Introduction: Preventing HIV and unintended pregnancies are key global health priorities. To inform product rollout and to understand attributes of future multipurpose prevention technologies (MPT) associated with preference and use, we evaluated three placebo delivery forms: daily oral tablets, a monthly vaginal ring, and two monthly intramuscular injections in TRIO, a five-month study among young Kenyan and South African women.
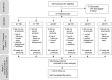
Methods: HIV-negative, sexually active, non-pregnant women aged 18 to 30 were enrolled and randomized to use each placebo delivery form for one month (stage 1). Then, participants chose one product to use for two additional months (stage 2). We assessed safety, product ranking, choice, and use. We examined demographic and behavioural correlates of choice and, reciprocally, unwillingness to use in the future with logistic regression models.
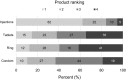
Results: 277 women enrolled, 249 completed stage 1 and 246 completed stage 2. Median age was 23 years, 49% were Kenyan and 51% were South African. Three participants became pregnant during the study and one participant HIV-seroconverted. There were 18 product-related adverse events, six tablets-related, 11 ring-related, and one injection-related. After trying each product, 85% preferred a TRIO product over condoms. Injections were chosen most (64%, 95% confidence interval (CI) 58%, 70%; p < 0.001), and by more South Africans than Kenyans (odds ratio (OR) 2.01, 95% CI: 1.17, 3.43; p = 0.01). There was no significant difference in choosing tablets versus ring (21%, 95% CI: 16%, 26% vs. 15%, 95% CI: 11%, 20%; p = 0.11). Tablet and ring adherence, based on direct observations and self-reports, improved over time. However, participants' self-reported use of tablets did not match objective data from the electronic dose monitoring device. Participants were fully compliant with injections.
Conclusion: In this population at risk for HIV and pregnancy, all participants agreed to choose and use a placebo MPT delivery form. A majority of participants preferred TRIO products to male condoms, an existing MPT. Injections were most liked and best used, however, they are years away from reaching the clinics. In the meantime, expanding the availability of tablets and giving access to rings can begin to fulfill the promise of choice for HIV prevention technologies and inform the development of suitable delivery forms as MPT.
Keywords: Africa; HIV prevention; contraception; end-user research; multipurpose prevention technologies; product preference.
© 2018 The Authors. Journal of the International AIDS Society published by John Wiley & sons Ltd on behalf of the International AIDS Society.
Figures
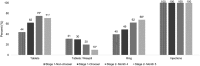
References
-
- Kott A. Rates of unintended pregnancy remain high in developing regions. Int Perspect Sex Reprod Health. 2011. Mar;37(1):46–7.
-
- HIV/AIDS JUNPo . Global AIDS Update 2016. Geneva, Switzerland; 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

