Titanium dioxide nanoparticle exposure alters metabolic homeostasis in a cell culture model of the intestinal epithelium and Drosophila melanogaster
- PMID: 29600885
- PMCID: PMC6157847
- DOI: 10.1080/17435390.2018.1457189
Titanium dioxide nanoparticle exposure alters metabolic homeostasis in a cell culture model of the intestinal epithelium and Drosophila melanogaster
Abstract
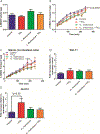
Nanosized titanium dioxide (TiO2) is a common additive in food and cosmetic products. The goal of this study was to investigate if TiO2 nanoparticles affect intestinal epithelial tissues, normal intestinal function, or metabolic homeostasis using in vitro and in vivo methods. An in vitro model of intestinal epithelial tissue was created by seeding co-cultures of Caco-2 and HT29-MTX cells on a Transwell permeable support. These experiments were repeated with monolayers that had been cultured with the beneficial commensal bacteria Lactobacillus rhamnosus GG (L. rhamnosus). Glucose uptake and transport in the presence of TiO2 nanoparticles was assessed using fluorescent glucose analog 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG). When the cell monolayers were exposed to physiologically relevant doses of TiO2, a statistically significant reduction in glucose transport was observed. These differences in glucose absorption were eliminated in the presence of beneficial bacteria. The decrease in glucose absorption was caused by damage to intestinal microvilli, which decreased the surface area available for absorption. Damage to microvilli was ameliorated in the presence of L. rhamnosus. Complimentary studies in Drosophila melanogaster showed that TiO2 ingestion resulted in decreased body size and glucose content. The results suggest that TiO2 nanoparticles alter glucose transport across the intestinal epithelium, and that TiO2 nanoparticle ingestion may have physiological consequences.
Keywords: Caco-2; GI tract; Lactobacillus rhamnosus; glucose; microbiome.
Figures
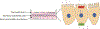




References
-
- Lomer MCE, Thompson RPH and Powell JJ. 2002. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proceedings of the Nutrition Society 61:123–130. - PubMed
-
- Robichaud CO, Uyar AE, Darby MR, Zucker LG and Wiesner MR. 2009. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ Sci Technol 43:4227–4233. - PubMed
-
- Xia T, Li N and Nel AE. 2009. Potential health impact of nanoparticles. Annu Rev Public Health 30:137–150. - PubMed
-
- Maynard AD. 2006. Nanotechnology: assessing the risks. Nano Today 1:22–33.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
