Potent 1,2,4-Triazino[5,6 b]indole-3-thioether Inhibitors of the Kanamycin Resistance Enzyme Eis from Mycobacterium tuberculosis
- PMID: 29601176
- PMCID: PMC6528465
- DOI: 10.1021/acsinfecdis.8b00074
Potent 1,2,4-Triazino[5,6 b]indole-3-thioether Inhibitors of the Kanamycin Resistance Enzyme Eis from Mycobacterium tuberculosis
Abstract
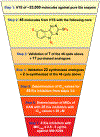
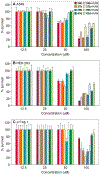
A common cause of resistance to kanamycin (KAN) in tuberculosis is overexpression of the enhanced intracellular survival (Eis) protein. Eis is an acetyltransferase that multiacetylates KAN and other aminoglycosides, rendering them unable to bind the bacterial ribosome. By high-throughput screening, a series of substituted 1,2,4-triazino[5,6 b]indole-3-thioether molecules were identified as effective Eis inhibitors. Herein, we purchased 17 and synthesized 22 new compounds, evaluated their potency, and characterized their steady-state kinetics. Four inhibitors were found not only to inhibit Eis in vitro, but also to act as adjuvants of KAN and partially restore KAN sensitivity in a Mycobacterium tuberculosis KAN-resistant strain in which Eis is upregulated. A crystal structure of Eis in complex with a potent inhibitor and CoA shows that the inhibitors bind in the aminoglycoside binding site snugly inserted into a hydrophobic cavity. These inhibitors will undergo preclinical development as novel KAN adjuvant therapies to treat KAN-resistant tuberculosis.
Keywords: aminoglycoside resistance; antitubercular agent; combination therapy; high-throughput screen; structure-activity relationship (SAR).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

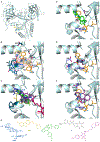

References
-
- (2016) Global Tuberculosis Report 2016, pp 1–211, World Health Organization.
-
- Unissa AN, Subbian S, Hanna LE, and Selvakumar N (2016) Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect., Genet. Evol 45, 474–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

