Measurable Residual Disease at Induction Redefines Partial Response in Acute Myeloid Leukemia and Stratifies Outcomes in Patients at Standard Risk Without NPM1 Mutations
- PMID: 29601212
- PMCID: PMC5959196
- DOI: 10.1200/JCO.2017.76.3425
Measurable Residual Disease at Induction Redefines Partial Response in Acute Myeloid Leukemia and Stratifies Outcomes in Patients at Standard Risk Without NPM1 Mutations
Abstract
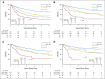
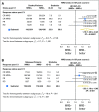
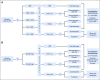
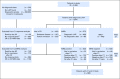
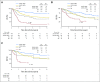
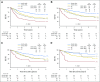
Purpose We investigated the effect on outcome of measurable or minimal residual disease (MRD) status after each induction course to evaluate the extent of its predictive value for acute myeloid leukemia (AML) risk groups, including NPM1 wild-type (wt) standard risk, when incorporated with other induction response criteria. Methods As part of the NCRI AML17 trial, 2,450 younger adult patients with AML or high-risk myelodysplastic syndrome had prospective multiparameter flow cytometric MRD (MFC-MRD) assessment. After course 1 (C1), responses were categorized as resistant disease (RD), partial remission (PR), and complete remission (CR) or complete remission with absolute neutrophil count < 1,000/µL or thrombocytopenia < 100,000/μL (CRi) by clinicians, with CR/CRi subdivided by MFC-MRD assay into MRD+ and MRD-. Patients without high-risk factors, including Flt3 internal tandem duplication wt/- NPM1-wt subgroup, received a second daunorubicin/cytosine arabinoside induction; course 2 (C2) was intensified for patients with high-risk factors. Results Survival outcomes from PR and MRD+ responses after C1 were similar, particularly for good- to standard-risk subgroups (5-year overall survival [OS], 27% RD v 46% PR v 51% MRD+ v 70% MRD-; P < .001). Adjusted analyses confirmed significant OS differences between C1 RD versus PR/MRD+ but not PR versus MRD+. CRi after C1 reduced OS in MRD+ (19% CRi v 45% CR; P = .001) patients, with a smaller effect after C2. The prognostic effect of C2 MFC-MRD status (relapse: hazard ratio [HR], 1.88 [95% CI, 1.50 to 2.36], P < .001; survival: HR, 1.77 [95% CI, 1.41 to 2.22], P < .001) remained significant when adjusting for C1 response. MRD positivity appeared less discriminatory in poor-risk patients by stratified analyses. For the NPM1-wt standard-risk subgroup, C2 MRD+ was significantly associated with poorer outcomes (OS, 33% v 63% MRD-, P = .003; relapse incidence, 89% when MRD+ ≥ 0.1%); transplant benefit was more apparent in patients with MRD+ (HR, 0.72; 95% CI, 0.31 to 1.69) than those with MRD- (HR, 1.68 [95% CI, 0.75 to 3.85]; P = .16 for interaction). Conclusion MFC-MRD can improve outcome stratification by extending the definition of partial response after first induction and may help predict NPM1-wt standard-risk patients with poor outcome who benefit from transplant in the first CR.
Figures





Comment in
-
Minimal Residual Disease Testing After Induction Chemotherapy for Acute Myeloid Leukemia: Moving Beyond Prognostication?J Clin Oncol. 2018 May 20;36(15):1463-1465. doi: 10.1200/JCO.2018.78.3266. Epub 2018 Apr 6. J Clin Oncol. 2018. PMID: 29624462 No abstract available.
References
-
- Wheatley K, Burnett AK, Goldstone AH, et al. : A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. Br J Haematol 107:69-79, 1999 - PubMed
-
- Schlenk RF, Benner A, Hartmann F, et al. : Risk-adapted postremission therapy in acute myeloid leukemia: Results of the German multicenter AML HD93 treatment trial. Leukemia 17:1521-1528, 2003 - PubMed
-
- Kern W, Haferlach T, Schoch C, et al. : Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: Data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood 101:64-70, 2003 - PubMed
-
- O’Donnell MR, Tallman MS, Abboud CN, et al. : Acute myeloid leukemia, version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15:926-957, 2017 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

