Analysis of the dose-sparing effect of adjuvanted Sabin-inactivated poliovirus vaccine (sIPV)
- PMID: 29601259
- PMCID: PMC6150041
- DOI: 10.1080/21645515.2018.1454571
Analysis of the dose-sparing effect of adjuvanted Sabin-inactivated poliovirus vaccine (sIPV)
Abstract
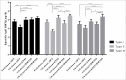
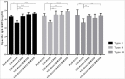
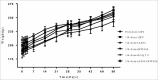
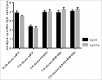
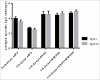
Sabin-based inactivated poliovirus vaccine(sIPV) is gradually replacing live-attenuated oral polio vaccine(OPV). Sabin-inactivated poliovirus vaccine(sIPV) has played a vital role in reducing economic burden of poliomyelitis and maintaining appropriate antibody levels in the population. However, due to its high cost and limited manufacturing capacity, sIPV cannot reach its full potential for global poliovirus eradication in developing countries. Therefore, to address this situation, we designed this study to evaluate the dose-sparing effects of AS03, CpG oligodeoxynucleotides (CpG-ODN) and polyinosinic:polycytidylic acid (PolyI:C) admixed with sIPV in rats. Our results showed that a combination of 1/4-dose sIPV adjuvanted with AS03 or AS03 with BW006 provides a seroconversion rate similar to that of full-dose sIPV without adjuvant and that, this rate is 5-fold higher than that of 1/4-dose sIPV without adjuvant after the first immunization. The combination of AS03 or AS03 with BW006 as an adjuvant effectively reduced sIPV dose by at least 4-fold and induced both humoral and cellular immune responses. Therefore, our study revealed that the combination of AS03 or AS03 with BW006 is a promising adjuvant for sIPV development.
Keywords: AS03; CpG ODN; adjuvant; polyi:C; sabin IPV.
Figures
References
-
- Home C. Laboratory surveillance for wild and vaccine-derived polioviruses--worldwide, January 2007-June 2008. Mmwr Morbidity & Mortality Weekly Report. 2008;57:967. - PubMed
-
- Roberts L. A one-two punch against polio. Petroleum Rock Mechanics. 2014;345:63–76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
