Efforts to Improve the Seasonal Influenza Vaccine
- PMID: 29601497
- PMCID: PMC6027170
- DOI: 10.3390/vaccines6020019
Efforts to Improve the Seasonal Influenza Vaccine
Abstract
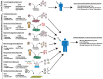
Influenza viruses infect approximately 20% of the global population annually, resulting in hundreds of thousands of deaths. While there are Food and Drug Administration (FDA) approved antiviral drugs for combating the disease, vaccination remains the best strategy for preventing infection. Due to the rapid mutation rate of influenza viruses, vaccine formulations need to be updated every year to provide adequate protection. In recent years, a great amount of effort has been focused on the development of a universal vaccine capable of eliciting broadly protective immunity. While universal influenza vaccines clearly have the best potential to provide long-lasting protection against influenza viruses, the timeline for their development, as well as the true universality of protection they afford, remains uncertain. In an attempt to reduce influenza disease burden while universal vaccines are developed and tested, many groups are working on a variety of strategies to improve the efficacy of the standard seasonal vaccine. This review will highlight the different techniques and technologies that have been, or are being, developed to improve the seasonal vaccination efforts against influenza viruses.
Keywords: egg-adaptation; influenza; seasonal vaccine; vaccine efficacy.
Conflict of interest statement
Duke University has filed for protection of the intellectual property utilizing dual-HA influenza viruses as vaccines described in this review.
Figures

References
-
- Shaw M.L., Palese P. Orthomyxoviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2013. pp. 1151–1185.
-
- World Health Organization (WHO) Influenza (Seasonal) [(accessed on 12 February 2018)]; Available online: http://www.who.int/mediacentre/factsheets/fs211/en/
-
- CDC Key Facts about Seasonal Flu Vaccine. [(accessed on 12 February 2018)]; Available online: https://www.cdc.gov/flu/protect/keyfacts.htm.
-
- CDC Quadrivalent Influenza Vaccine. [(accessed on 12 February 2018)]; Available online: https://www.cdc.gov/flu/protect/vaccine/quadrivalent.htm.
-
- CDC Selecting Viruses for the Seasonal Influenza Vaccine. [(accessed on 12 February 2018)]; Available online: https://www.cdc.gov/flu/about/season/vaccine-selection.htm.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

