Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial
- PMID: 29601570
- PMCID: PMC5877834
- DOI: 10.1371/journal.pmed.1002547
Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial
Abstract
Background: As the number of HIV-infected women initiating lifelong antiretroviral therapy (ART) during pregnancy increases globally, concerns have emerged regarding low levels of retention in HIV services and suboptimal adherence to ART during the postpartum period. We examined the impact of integrating postpartum ART for HIV+ mothers alongside infant follow-up within maternal and child health (MCH) services in Cape Town, South Africa.
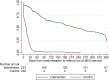
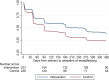
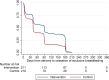
Methods and findings: We conducted a randomised trial among HIV+ postpartum women aged ≥18 years who initiated ART during pregnancy in the local antenatal care clinic and were breastfeeding when screened before 6 weeks postpartum. We compared an integrated postnatal service among mothers and their infants (the MCH-ART intervention) to the local standard of care (control)-immediate postnatal referral of HIV+ women on ART to general adult ART services and their infants to separate routine infant follow-up. Evaluation data were collected through medical records and trial measurement visits scheduled and located separately from healthcare services involved in either arm. The primary trial outcome was a composite endpoint of women's retention in ART care and viral suppression (VS) (viral load < 50 copies/ml) at 12 months postpartum; secondary outcomes included duration of any and exclusive breastfeeding, mother-to-child HIV transmission, and infant mortality. Between 5 June 2013 and 10 December 2014, a total of 471 mother-infant pairs were enrolled and randomised (mean age, 28.6 years; 18% nulliparous; 57% newly diagnosed with HIV in pregnancy; median duration of ART use at randomisation, 18 weeks). Among 411 women (87%) with primary endpoint data available, 77% of women (n = 155) randomised to the MCH-ART intervention achieved the primary composite outcome of retention in ART services with VS at 12 months postpartum, compared to 56% of women (n = 117) randomised to the control arm (absolute risk difference, 0.21; 95% CI: 0.12-0.30; p < 0.001). The findings for improved retention in care and VS among women in the MCH-ART intervention arm were consistent across subgroups of participants according to demographic and clinical characteristics. The median durations of any breastfeeding and exclusive breastfeeding were longer in women randomised to the intervention versus control arm (6.9 versus 3.0 months, p = 0.006, and 3.0 versus 1.4 months, p < 0.001, respectively). For the infants, overall HIV-free survival through 12 months of age was 97%: mother-to-child HIV transmission was 1.2% overall (n = 4 and n = 1 transmissions in the intervention and control arms, respectively), and infant mortality was 1.9% (n = 6 and n = 3 deaths in the intervention and control arms, respectively), and these outcomes were similar by trial arm. Interpretation of these findings should be qualified by the location of this study in a single urban area as well as the self-reported nature of breastfeeding outcomes.
Conclusions: In this study, we found that integrating ART services into the MCH platform during the postnatal period was a simple and effective intervention, and this should be considered for improving maternal and child outcomes in the context of HIV.
Trial registration: ClinicalTrials.gov NCT01933477.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 infection. N Engl J Med. 2016; 374(8):761–70. doi: 10.1056/NEJMra1505256 - DOI - PubMed
-
- Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726–37. doi: 10.1056/NEJMoa1511691 - DOI - PMC - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection 2nd edition Geneva: World Health Organization; 2016. - PubMed
-
- Joint United Nations Programme on HIV/AIDS. Get on the fast-track: the life-cycle approach to HIV Geneva: Joint United Nations Programme on HIV/AIDS; 2016.
-
- Huntington S, Thorne C, Newell ML, Anderson J, Taylor GP, Pillay D, et al. The risk of viral rebound in the year after delivery in women remaining on antiretroviral therapy. AIDS. 2015;29(17):2269–78. doi: 10.1097/QAD.0000000000000826 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

