Brugia malayi infection in ferrets - A small mammal model of lymphatic filariasis
- PMID: 29601572
- PMCID: PMC5895066
- DOI: 10.1371/journal.pntd.0006334
Brugia malayi infection in ferrets - A small mammal model of lymphatic filariasis
Abstract
Background: The lack of effective short-course therapies for treatment of the adult stage of filarial worms is a major limitation in the global effort to eliminate lymphatic filariasis. Studies using current small mammal models of lymphatic filariasis are limited by difficulties in quantifying adult worm numbers and in assessing lymphatic anatomy and function.
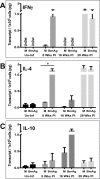
Methodology/principal findings: Here, we re-established Brugia malayi infection of ferrets as a model for lymphatic filariasis and demonstrated parasitological, immunological, and histological parallels with human infection. Subcutaneous injection of L3 larvae into a hind-footpad resulted in a mean of 18 adult worms recovered 16 weeks post-infection, primarily from the draining inguinal and femoral lymphatics of the injected limb. Infected ferrets developed microfilaremia, with patency lasting from 12-26 weeks post-infection. Quantitative PCR assessing cytokine transcription by antigen-stimulated lymph node cells demonstrated a mixed Th1/Th2 response occurring during early infection. Immunoregulation with production of down-regulatory cytokine IL-10 occurred just prior to peak microfilaremia. Histological analysis revealed progressive inflammation of the lymphatic vessel walls, with intimal thickening and disorganization of collagen fibers. Inflammation was observed as early as 8 weeks post-infection and extended into the perivascular and subcutaneous tissues by 16 weeks post-infection. Finally, we developed a novel ferret PET/CT lymphoscintigraphy method demonstrating substantial changes in lymphatic anatomy and function as early as 3 weeks post-infection, with progression over the course of infection.
Conclusions/significance: B. malayi infection of ferrets is a robust model of human lymphatic filariasis that can be utilized to study efficacy of novel antifilarial agents against adult worms residing within lymphatic vessels. In conjunction with PET/CT lymphoscintigraphy, this model can also be used to investigate pathogenesis of lymphatic dysfunction in lymphatic filariasis and efficacy of medications aimed at reversing lymphatic dysfunction after clearance of adult worms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Ramaiah KD, Ottesen EA. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis. 2014;8(11):e3319 doi: 10.1371/journal.pntd.0003319 ; PubMed Central PMCID: PMCPMC4239120. - DOI - PMC - PubMed
-
- Taylor MJ, Hoerauf A, Townson S, Slatko BE, Ward SA. Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis. Parasitology. 2014;141(1):119–27. doi: 10.1017/S0031182013001108 ; PubMed Central PMCID: PMCPMC3884836. - DOI - PMC - PubMed
-
- Mackenzie CD, Geary TG. Addressing the current challenges to finding new anthelminthic drugs. Expert Rev Anti Infect Ther. 2013;11(6):539–41. doi: 10.1586/eri.13.49 . - DOI - PubMed
-
- Geary TG, Mackenzie CD. Progress and challenges in the discovery of macrofilaricidal drugs. Expert Rev Anti Infect Ther. 2011;9(8):681–95. doi: 10.1586/eri.11.76 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

