The importance of greater speed in drug development for advanced malignancies
- PMID: 29601671
- PMCID: PMC5943431
- DOI: 10.1002/cam4.1454
The importance of greater speed in drug development for advanced malignancies
Abstract
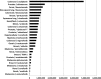
It takes on average 6-12 years to develop new anticancer drugs from discovery to approval. Effective new agents prolong survival. To demonstrate the importance of rapid drug approval, we calculated life-years potentially saved if selected agents were approved more rapidly. As illustrative examples, we used 27 trials documenting improvements in survival. We multiplied improvement in median survival by numbers of patients dying annually and multiplied this by number of years from drug discovery until approval. For every year by which time to drug approval could have been shortened, there would have been a median number of life-years potentially saved of 79,920 worldwide per drug. Median number of life-years lost between time of drug discovery and approval was 1,020,900 per example. If we were able to use available opportunities to decrease the time required to take a drug from discovery to approval to 5 years, the median number of life-years saved per example would have been 523,890 worldwide. Various publications have identified opportunities to speed drug development without sacrificing patient safety. While many investigational drugs prove to be ineffective, some significantly prolong survival and/or reduce suffering. These illustrative examples suggest that a substantial number of life-years could potentially be saved by increasing the efficiency of development of new drugs for advanced malignancies.
Keywords: Cancer; drug approval; drug development; life-years saved.
© 2018 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- Dickson, M. , and Gagnon J. P.. 2004. The cost of new drug discovery and development. Discov. Med. 4:172–179. - PubMed
-
- Pammolli, F. , Magazzini L., and Riccaboni M.. 2011. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug. Discov. 10:428–438. - PubMed
-
- Langreth, R. , and Staley O.. 2015. Faster FDA spurs cancer breakthroughs. Bloomberg Business Available at http://www.bloomberg.com/news/articles/2015-03-24/faster-fda-spurs-cance.... March 24.
-
- Flashback: FDA Drug Approvals 2013. Drugscom. Available at http://www.drugs.com/slideshow/new-and-unique-drugs-approved-in-2013-107... (accessed July 05, 2015).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

