Sequential intracellular release of water-soluble cargos from Shell-crosslinked polymersomes
- PMID: 29601932
- PMCID: PMC6008218
- DOI: 10.1016/j.jconrel.2018.03.027
Sequential intracellular release of water-soluble cargos from Shell-crosslinked polymersomes
Abstract
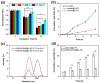
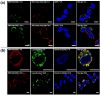
Polymer vesicles, i.e. polymersomes (PS), present unique nanostructures with an interior aqueous core that can encapsulate multiple independent cargos concurrently. However, the sequential release of such co-loaded actives remains a challenge. Here, we report the rational design and synthesis of oxidation-responsive shell-crosslinked PS with capability for the controlled, sequential release of encapsulated hydrophilic molecules and hydrogels. Amphiphilic brush block copolymers poly(oligo(ethylene glycol) methyl ether methacrylate)-b-poly(oligo(propylene sulfide) methacrylate) (POEGMA-POPSMA) were prepared to fabricate PS via self-assembly in aqueous solution. As a type of unique drug delivery vehicle, the interior of the PS was co-loaded with hydrophilic molecules and water-soluble poly(N-isopropylacrylamide) (PNIPAM) conjugates. Due to the thermosensitivity of PNIPAM, PNIPAM conjugates within the PS aqueous interior underwent a phase transition to form hydrogels in situ when the temperature was raised above the lower critical solution temperature (LCST) of PNIPAM. Via control of the overall shell permeability by oxidation, we realized the sequential release of two water-soluble payloads based on the assumption that hydrogels have much smaller membrane permeability than that of molecular cargos. The ability to control the timing of release of molecular dyes and PNIPAM-based hydrogels was also observed within live cells. Furthermore, leakage of hydrogels from the PS was effectively alleviated in comparison to molecular cargos, which would facilitate intracellular accumulation and prolonged retention of hydrogels within the cell cytoplasm. Thus, we demonstrate that the integration of responsive hydrogels into PS with crosslinkable membranes provides a facile and versatile technique to control the stability and release of water-soluble cargos for drug delivery purposes.
Keywords: Oxidation-responsive; Polymersomes; Self-assembly; Sequential release; Shell crosslinking.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
F.D., S.B., S.Y., and E.A.S. declare that they have no conflicts of interest.
Figures






References
-
- Discher DE, Eisenberg A. Polymer Vesicles. Science. 2002;297(5583):967–973. - PubMed
-
- Zhu Y, et al. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Progress in Polymer Science. 2017;64(Supplement C):1–22.
-
- Vasdekis AE, et al. Vesicle Photonics. Annual Review of Materials Research. 2013;43(1):283–305.
-
- Thambi T, Park JH, Lee DS. Stimuli-responsive polymersomes for cancer therapy. Biomaterials Science. 2016;4(1):55–69. - PubMed
-
- Scott EA, Karabin NB, Augsornworawat Overcoming Immune Dysregulation with Immunoengineered Nanobiomaterials. Annual Review of Biomedical Engineering. 2017;19(1):57–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

